Back to Journals » Journal of Inflammation Research » Volume 17
The Level of Fibrinogen-to-Albumin Ratio Was Associated with the Severity of Cerebral Small Vessel Disease in Patients with Transient Ischemic Attack
Authors Liu C, Chen L, Sun D, Guo Y, Zhu H, Li L, Sun S, He G, Cheng Y
Received 25 July 2024
Accepted for publication 27 November 2024
Published 5 December 2024 Volume 2024:17 Pages 10441—10451
DOI https://doi.org/10.2147/JIR.S488600
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Changxia Liu,1,* Li Chen,2,* Dingming Sun,1 Yan Guo,1 Honghong Zhu,3 Lei Li,1 Shifu Sun,1 Guojun He,1 Yongqing Cheng1
1Department of Neurology, The Yancheng Clinical College of Xuzhou Medical University, The First People’s Hospital of Yancheng, Yancheng, Jiangsu Province, 224000, People’s Republic of China; 2Department of Ophtalmology, Funing People’s Hospital, Yancheng, Jiangsu Province, 224000, People’s Republic of China; 3Department of Rheumatology and Immunology, The Yancheng Clinical College of Xuzhou Medical University, The First People’s Hospital of Yancheng, the First People’s Hospital of Yancheng, Yancheng, Jiangsu Province, 224000, People’s Republic of China
*These authors contributed equally to this work
Coresspondence: Guojun He; Yongqing Cheng
Department of Neurology, The Yancheng Clinical College of Xuzhou Medical University, The First People’s Hospital of Yancheng, Yancheng, Jiangsu Province, 224000, People’s Republic of China
, Tel +86-15251131959
, +86-13851190252
, Email [email protected]; [email protected]
Purpose: Inflammation plays a role in cerebral small vessel disease (CSVD) pathophysiology. This study aimed to explore the association of the fibrinogen-to-albumin ratio (FAR), a novel inflammatory marker, with CSVD burden in patients with transient ischemic attack (TIA).
Patients and Methods: From October 1, 2022, to November 30, 2023, continuous patients with TIA were recruited in the study. The total CSVD burden score and modified total CSVD burden score were used to assess the severity of CSVD. Multivariable regression analysis was used to explore the correlation between the FAR and CSVD in TIA patients.
Results: A total of 455 participants were recruited, of whom 225 (48.35%), according to the total CSVD burden score, and 181 (40.67%), according to the modified CSVD burden score were finally identified as moderate-severe CSVD. Spearman correlation analysis showed that levels of FAR correlated with the total CSVD (r=0.392, P< 0.001) and the modified total CSVD burden scores (r=0.379, P< 0.001). Multivariable logistic regression analysis showed that FAR was independently associated with moderate-severe CSVD, both as a continuous variable and as a tertile variable (P< 0.001).
Conclusion: The level of FAR on admission was independently associated with the severity of CSVD in patients with TIA.
Keywords: cerebral small vessel disease, fibrinogen-to-albumin ratio, transient ischemic attack, inflammatory marker
Introduction
Cerebral small vessel disease (CSVD) is a common cerebrovascular disease with an extremely high incidence in the elderly population, affecting 5% of people aged over 50 and almost 100% of people aged over 90.1,2 The diagnosis of CSVD mainly depends on imaging findings, including recent small subcortical infarct, white matter hyperintensities (WMHs), lacunes, cerebral microbleeds (CMBs), enlarged perivascular spaces (EPVS), and brain atrophy.2 CSVD has been proved to cause dementia, gait dysfunction, and mood disorders and is associated with the recurrence and prognosis of ischemic stroke and TIA.3,4
Although the underlying mechanism of CSVD is still unclear, increasing evidence suggests that inflammation might play a crucial role. Many systemic inflammation markers, such as C-reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor α (TNF-α), and vascular endothelial growth factor (VEGF) have been reported to be associated with CSVD.5 As a new inflammatory marker derived from fibrinogen and albumin, the FAR has been reported to be associated with a variety of diseases, including cardiovascular diseases, tumors, and stroke.6–8 However, the correlation between FAR and CSVD is still unclear. We designed this prospective cross-sectional study to explore the relationship between the level of FAR on admission and the severity of CSVD in patients with TIA.
Materials and Methods
Study Design and Participants
From October 2022 to November 2023, patients with first-ever TIA admitted to inpatient department of the First People’s Hospital of Yancheng were consecutively enrolled in this study. The Ethics Committee of the First People’s Hospital of Yancheng approved this study. It was conducted in accordance with the principles of the Declaration of Helsinki. All patients themselves provided written informed consent.
Inclusion criteria included (1) first TIA, (2) admission within 7 days of onset, (3) over 18 years old. The exclusion criteria were as follows: (1) a history of TIA or ischemic stroke, (2) contraindications to MRI examination, (3) other diseases with MRI abnormalities, such as brain trauma, brain tumors, multiple sclerosis, cerebral hemorrhage, central nervous system infection, hydrocephalus, or autoimmune encephalitis, (4) with diseases which might affect inflammatory conditions, such as blood disease, acute infection, tumors, severe liver or kidney disease and autoimmune diseases, (5) use of any anticoagulants within one month, (6) incomplete MRI data.
The diagnosis of TIA depends on symptom duration and the absence of acute cerebral infarction on DWI. Transient focal neurologic deficit symptoms should coincide with corresponding cerebrovascular regions and can be completely recovered, lasting less than 24 hours.9
Baseline Data Collection
The baseline data were collected by a trained physician on admission using a standardized protocol. We collected demographic data (age, gender), medical history (hypertension, diabetes, hyperlipidemia, atrial fibrillation, coronary heart disease), pharmacological treatment history (antiplatelet, statin, antihypertension and antidiabetes), history of smoking and drinking, and clinical assessment (blood pressure, ABCD2 score).
Fasting venous blood was drawn from all patients the morning after admission. Laboratory data including fibrinogen, D-Dimmer, albumin, total cholesterol (TC), triglyceride (TG), fasting plasma glucose (FPG), white blood cell count, and neutrophil count. FAR was calculated by the formula fibrinogen (g/L) / albumin (g/L). Two technicians who were blinded to the clinical data processed all blood samples in the laboratory department of our hospital.
MRI Acquisition and Assessment
All participants underwent brain MRI including T1-weighted, T2-weighted, T2 fluid-attenuated inversion recovery (FLAIR), diffusion weighted imaging (DWI) and susceptibility-weighted imaging (SWI) sequences within 3 days of admission by a 3.0T MRI scanner.
Two trained neuroradiologists blinded to all clinical data processed the MRI imaging data. WMH, including the periventricular and deep WMH, were evaluated based on T2 and Flair sequences by the Fazekas rating scale. Cerebral microbleeds (CMBs) were defined as circular-like low-density regions of 2–10 mm in size on the SWI sequence. Lacunae are round or ovoid fluid-filled cavities in the cortex that resemble cerebrospinal fluid (CSF) signals on T1, T2, or Flair sequences, ranging from 3 to 20mm in diameter. Enlarged perivascular spaces (EPVS) are linear round or oval spaces less than 3mm in diameter, similar to the signal of CSF. EPVS are commonly found in the basal ganglia and subcortical areas.2 We assessed the severity of CSVD using the total CSVD burden score proposed by Staals et al and the modified total CSVD burden score designed by Rothwell et al10,11 Both of them include the four CSVD-associated MRI markers described above. The total CSVD burden score ranges from 0 to 4. One point was allocated for the presence of each of the following MRI markers: the presence of lacunae or CMBs (one point for each); moderate-to-severe WMH including PV-WMH Fazekas 3 or deep-WMH Fazekas ≥2; and moderate-to-severe basal ganglia EPVS (BG-EPVS) (N≥11). While the modified total CSVD burden score ranges from 0 to 6. One point was assigned to the presence of lacunes, CMB burden (N 1–4), severe BG-EPVS (N>20), modified WMH burden (total WMH grade 3–4), and two points were allocated for CMB burden (N≥5) and modified WMH burden (total WMH grade 5–6).12 All patients were divided into no-mild (total CSVD burden score 0–1 or modified total CSVD burden score 0–2) or moderate-moderate (total CSVD burden score 2–4 or modified total CSVD burden score 3–6) subgroups according to the severity of CSVD.
Statistical Analysis
Statistical analyses were performed by SPSS version 23.0 (IBM, New York, NY, USA). Continuous variables are presented as the mean ± standard deviation or median (interquartile range [IQR]), and categorical variables are presented as numbers (percentages). All patients were categorized into four subgroups according to quartiles of FAR and divided into two subgroups named non-mild CSVD and moderate-severe CSVD subgroups based on the total CSVD burden score and modified total CSVD burden score. The Chi-square test or Fisher’s exact test was used for categorical variables (such as sex and medical history), one-way ANOVA, variance analysis, the Mann–Whitney U-test, or the Kruskal–Wallis test was used for continuous variables (such as age). The relationship between FAR and total CSVD burden score or modified total CSVD burden score was analyzed by spearman’s rank correlation. Univariate and multivariate logistic regression analyses were performed to identify the relationship between levels of FAR and the severity of CSVD. Univariate regression analysis was used to screen the risk factors of moderate-severe CSVD. To mitigate potential confounders, we constructed three regression models: an unadjusted model, a model adjusted for age and sex, and a multivariate adjusted model with further adjustment for all the pharmacological treatment history and other variables with a p < 0.1 in the univariate analysis. An association was indicated as the odds ratio (OR) or adjusted odds ratio (aOR) with the 95% confidence interval (CI). Statistical significance was defined as a two-sided P-value of < 0.05.
Results
Comparison of Characteristics Between FAR Quartiles
From September 2022 to October 2023, A total of 558 TIA patients were consecutively screened in this study. 103 patients were excluded for various reasons according to the exclusion criteria (Figure 1). Finally, 455 participants were recruited in the study with a median FAR of 8.006, a median age of 63 years, and 296 (65.05%) were male. The FAR was stratified by quartile, and the quartile levels were as follows: Quartile 1 (FAR≤6.65), Quartile 2 (6.66≤FAR<8.02), Quartile 3 (8.02≤FAR<9.77), Quartile 4 (9.77≤FAR). The results showed that significant differences appeared in the ABCD2 score, proportion of previous statin treatment, total CSVD burden, modified total CSVD burden, and levels of fibrinogen and albumin (all P <0.001). However, there is no significant difference in other characteristics (Table 1). The inter-observer variability coefficients for assessing of the total and modified total CSVD burden score were found to be 16.7% and 17.4%, respectively, indicating high agreement among the radiologists. The intra-observer variability coefficients for the repeated measurements of the total and modified total CSVD burden score 9.7% and 10.1%, respectively, suggesting high reproducibility within the same radiologist.
 |
Table 1 Bseline Characteristics Among Patients According to Quartiles of FAR |
 |
Figure 1 Flowchart of the study. |
Comparison of Characteristics Between Patients with Different Severity of CSVD
We divided the patients into non-mild and moderate-severe subgroups based on the total CSVD and modified total CSVD burden respectively. Compared with the non-mild subgroup, patients in the moderate-severe subgroup had higher levels of FAR,age, ABCD2 score, systolic blood pressure, FPG, D-dimer, fibrinogen, albumin, as well as a higher proportion of hypertension, previous antihypertension treatment and previous antiplatelet treatment (all P<0.05), either total CSVD burden score or modified total CSVD burden score was used as the classification standard. In addition, the moderate-severe subgroup also had a higher proportion of diabetes (P=0.013) when the modified CSVD score was used as a grouping criterion (Table 2).
 |
Table 2 Demographic and Clinical Characteristics According to the Severity of CSVD |
Correlation Between FAR and Total CSVD Burden or Modified Total CSVD Burden Scores
Spearman correlation analysis was used to explore the correlation between FAR and CSVD score or modified CSVD score. The results showed that FAR was significantly correlated with CSVD burden and modified CSVD burden scores (Figures 2 and 3). The results show that FAR is significantly correlated with both CSVD (r=0.392, P<0.001) and modified CSVD (r=0.379, P<0.001) when used as a continuous variable.
 |
Figure 2 Spearman correlation analysis between FAR and the total CSVD burden score. |
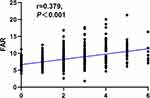 |
Figure 3 Spearman correlation analysis between FAR and the modified total CSVD burden score. |
Associations of FAR with the Severity of CSVD
The results of logistic regression analyses for moderate–severe CSVD with FAR as continuous variables and quartiles were displayed in Tables 3 and 4. Fibrinogen and albumin were eliminated from the multivariate regression model due to collinearity with FAR. The multivariate regression analysis, which was adjusted for gender, age, all the pharmacological treatment history and other variables with P<0.1 in univariable regression analysis, showed that FAR (OR=1.477, 95% CI=1.323–1.649 for total CSVD burden and OR=1.480, 95% CI=1.329–1.649 for modified total CSVD burden, respectively) as a continuous variable was independently associated with moderate–severe CSVD. Similarly, when FAR was included as quartiles in the multivariable regression analysis, the results indicated that compared with the first quartile of FAR, the other quartiles could significantly increase the risk of moderate-severe CSVD (all P <0.001).
 |
Table 3 Multivariate Regression Analyses for Moderate–Severe CSVD with FAR as Continuous Variables |
 |
Table 4 Multivariate Regression Analyses for Moderate–Severe CSVD with FAR as Quartiles |
Discussion
To our knowledge, insufficient literature has investigated the relationship between the level of FAR and CSVD in the TIA population. This study is the first prospective cross-sectional study to report an association between FAR and CSVD in patients with TIA. The results showed that a high level of FAR was independently associated with a higher total and modified total CSVD burden scores, even after adjusting for variables such as age, sex, and pharmacological treatment history, and could also increase the risk of moderate-severe CSVD. Consistent with previous reports, we also found that age and history of hypertension were associated with CSVD.13
CSVD is a group of pathological processes that affect cerebral small arteries, arterioles, capillaries, and cerebral venules.5 Epidemiological data suggest that CSVD can cause dementia, gait dysfunction, and mood disorders and is associated with the recurrence and prognosis of ischemic stroke and TIA.3,4,14 As an overall assessment scale for CSVD, the total CSVD burden score has also been reported to be correlated to stroke. According to a cohort study with a mean follow-up of 7.2 years, a higher total CSVD burden score could escalate the risk of stroke, dementia, and death.15 Furthermore, the total CSVD burden score could also predict the recurrence and prognosis of ischemic stroke/TIA.16
The underlying pathogenesis of CSVD is unclear, but Numerous animal and epidemiological studies have shown that inflammation is associated with vascular endothelial injury, oxidative stress, and hypoperfusion, all of which can induce CSVD.17–20 Both systemic inflammatory markers and vascular inflammatory markers have been demonstrated to have certain predictive effects on CSVD.5 Low et al ‘s systematic review reported that vascular inflammatory response mainly plays a role in the formation of CSVD in the basal ganglia, which is different from systemic inflammation, which mainly plays a role in cerebral amyloid angiopathy (CAA) related cerebral lobe and semi-oval area. Therefore, the association of systemic inflammatory markers and vascular inflammatory markers with CSVD may originate from different mechanisms. However, some studies also reported inconsistent results.19
As mentioned above, fibrinogen is a glycoprotein produced by the liver that plays a role in the inflammatory response and is considered a systemic inflammatory marker. A previous meta-analysis of 31 prospective studies showed that elevated fibrinogen was significantly associated with ischemic stroke risk.21 In addition, high levels of fibrinogen might also correlate to poor prognosis of cardiovascular disease, ischemic stroke, and TIA. Hou et al reported in a large cohort study involving 10518 patients that high levels of baseline and 90-day fibrinogen levels were independently associated with poor outcomes in patients with ischemic stroke and TIA.22 However, the correlation between fibrinogen and CSVD is still controversial. Some research has reported fibrinogen levels were significantly and independently associated with WMH.23,24 A study based on patients from the Framingham Heart Study found a significant association between fibrinogen and PVS in the basal ganglia but not in the centrum semiovale.25 However, some other studies have failed to find a correlation between fibrinogen and CSVD.26,27 Albumin is the most abundant serum protein synthesized in the liver, which has anti-platelet and anti-inflammatory effects. Several studies have explored the potential association between albumin and stroke. According to a recent large prospective cohort study, low level of albumin could effectively predict the poor prognosis and mortality of AIS and TIA patients at 3 months and 1 year.28 In addition, preclinical studies have shown that appropriate albumin treatment after ischemic stroke can effectively reduce brain edema and infarct volume, thereby improving the prognosis.29 Unfortunately, clinical studies in humans have not yielded consistent results.30 Meanwhile, the correlation between CSVD and albumin is unclear because few relevant studies exist.
FAR, a new inflammatory marker converted from fibrinogen and albumin has been reported to be an effective predictor of the prognosis of tumors and coronary heart disease, with better sensitivity and specificity than fibrinogen or albumin alone.31 At present, several publications have confirmed that high levels of FAR were associated with poor prognosis of tumors, such as breast and digestive system tumors.30,32 In addition, elevated FAR has also been reported to be independently associated with the severity and poor prognosis of coronary heart disease.33–35 A few recent studies have found possible correlation between FAR and stroke. A cohort study involving 264 patients with acute pontine infarction demonstrated that high levels of FAR could effectively predict poor prognosis after 3 months.35 A consistent conclusion was reached in another study of patients with acute lacunar stroke.36 Moreover, recent studies have found that high levels of FAR could increase the risk of hemorrhagic transformation after acute ischemic stroke.37,38 This study for the first time pointed out that FAR was associated with the severity of CSVD in patients with TIA.
The underlying mechanism of the potential association was unclear. Still, we speculated that inflammatory response played a central role. Meanwhile, high levels of FAR were often accompanied by high fibrinogen and low albumin, which might also play a potential role. Since CSVD is associated with poor prognosis of TIA, if the effectiveness of FAR in predicting CSVD in TIA patients is fully confirmed, it may open the door to various brain protective measures for TIA. Due to the close correlation between FAR and inflammation, appropriate treatment for high-risk patients to reduce inflammation may reduce the severity of CSVD in TIA patients and improve the prognosis. In addition, the related treatment strategies of reducing fibrinogen or increasing albumin may also become another new target for TIA treatment. However, at this stage, because the exact causal relationship between FAR and CSVD cannot be clarified, it seems that it is not enough to change the direction of treatment. This study provided epidemiological evidence for the association between FAR and CSVD in TIA patients. However, some limitations should be noted. First, this study was cross-sectional; the exact causal relationship between FAR and CSVD cannot be clarified. Second, due to the limitation of sample size, we did not perform subgroup analysis according to the type of CSVD, and further subgroup analysis would be more conducive to exploring the potential pathogenesis. Third, it is tough to include all risk factors associated with CSVD. In this study, some potential risk factors including C-reactive protein were not included in the analysis, which might have a certain impact on the final result. Finally, this is a single-center study with a limited sample size, which still needs to be further verified by multi-center and large-sample studies.
Conclusion
Our study shows that FAR levels are suggestively associated with the severity of CSVD in patients with TIA, independent of variables such as pharmacological treatment history, age and sex, with an unclear mechanism. The results need to be further verified by multi-center and large-sample studies. Further elucidating the mechanism may provide a new target for the treatment of CSVD.
Data Sharing Statement
We declare that all the data in this article are authentic, valid, and available from Yongqing Cheng for reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of The First People’s Hospital of Yancheng. This study was in accordance with the Declaration of Helsinki. Informed consent was obtained from all participants or their families before this study.
Acknowledgments
We express our gratitude to all the patients who participated in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Science and Technology Development Program Project of Yancheng [YK2021026].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689–701. doi:10.1016/S1474-4422(10)70104-6
2. Cannistraro RJ, Badi M, Eidelman BH, Dickson DW, Middlebrooks EH, Meschia JF. CNS small vessel disease. Neurology. 2019;92(24):1146–1156. doi:10.1212/WNL.0000000000007654
3. Chen H, Pan Y, Zong L, et al. Cerebral small vessel disease or intracranial large vessel atherosclerosis may carry different risk for future strokes. Stroke Vasc Neurol. 2020;5(2):128–137. doi:10.1136/svn-2019-000305
4. Ikeda S, Yakushiji Y, Tanaka J, et al. Hypertension, cerebral Amyloid, aGe Associated Known neuroimaging markers of cerebral small vessel disease Undertaken with stroke REgistry (HAGAKURE) prospective cohort study: baseline characteristics and association of cerebral small vessel disease with prognosis in an ischemic stroke cohort. Front Aging Neurosci. 2023;15:1117851. doi:10.3389/fnagi.2023.1117851
5. Wan S, Dandu C, Han G, et al. Plasma inflammatory biomarkers in cerebral small vessel disease: a review. CNS Neurosci Ther. 2023;29(2):498–515. doi:10.1111/cns.14047
6. Zhen Y, Tingyi H, Wang J, et al. Systemic immune-inflammation index as a potential biomarker of cardiovascular diseases: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;9:933913. doi:10.3389/fcvm.2022.933913
7. Bailey-Whyte M, Minas TZ, Dorsey TH, Smith CJ, Loffredo CA, Ambs S. Systemic Inflammation Indices and Association with Prostate Cancer Survival in a Diverse Patient Cohort. Cancers. 2023;15(6):1869. doi:10.3390/cancers15061869
8. Huang Y-W, Yin X-S, Zong-Ping L. Association of the systemic immune-inflammation index (SII) and clinical outcomes in patients with stroke: a systematic review and meta-analysis. Front Immunol. 2022;13:1090305. doi:10.3389/fimmu.2022.1090305
9. Amin HP, Madsen TE, Bravata DM, et al. Diagnosis, Workup, Risk Reduction of Transient Ischemic Attack in the Emergency Department Setting: a Scientific Statement From the American Heart Association. Stroke. 2023;54(3):e109–e121. doi:10.1161/STR.0000000000000418
10. Staals J, Makin SD, Doubal FN, Dennis MS, Wardlaw JM. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology. 2014;83(14):1228–1234. doi:10.1212/WNL.0000000000000837
11. Kai Lau K, Linxin L, Schulz U, et al. Total small vessel disease score and risk of recurrent stroke. Neurology. 2017;88(24):2260–2267. doi:10.1212/WNL.0000000000004042
12. Jiang L, Cai X, Yao D, et al. Association of inflammatory markers with cerebral small vessel disease in community-based population. J Neuroinflammation. 2022;19(1):106. doi:10.1186/s12974-022-02468-0
13. Hainsworth AH, Hugh S, Schneider JA. Cerebral Small Vessel Disease, Hypertension, and Vascular Contributions to Cognitive Impairment and Dementia. Hypertension. 2024;81(1):75–86. doi:10.1161/HYPERTENSIONAHA.123.19943
14. Debette S, Schilling S, Duperron M-G, Larsson SC, Markus HS. Clinical Significance of Magnetic Resonance Imaging Markers of Vascular Brain Injury. JAMA Neurol. 2019;76(1):81–94. doi:10.1001/jamaneurol.2018.3122
15. Pinar Yilmaz MKI, Wiro JN, Arfan Ikram M, Vernooij MW, Vernooij MW. Practical Small Vessel Disease Score Relates to Stroke, Dementia, and Death. Stroke. 2018;49(12):2857–2865. doi:10.1161/STROKEAHA.118.022485
16. Sung P-S, Lee K-P, Lin P-Y, et al. Factors Associated with Cognitive Outcomes After First-Ever Ischemic Stroke: the Impact of Small Vessel Disease Burden and Neurodegeneration. J Alzheimers Dis. 2021;83(2):569–579. doi:10.3233/JAD-210587
17. Inoue Y, Shue F, Guojun B, Kanekiyo T. Pathophysiology and probable etiology of cerebral small vessel disease in vascular dementia and Alzheimer’s disease. Mol Neurodegener. 2023;18(1):46. doi:10.1186/s13024-023-00640-5
18. Low A, Mak E, Rowe JB, Markus HS, O’Brien JT. Inflammation and cerebral small vessel disease: a systematic review. Ageing Res Rev. 2019;53:100916. doi:10.1016/j.arr.2019.100916
19. Del Cuore A, Pacinella G, Riolo R, Tuttolomondo A. The Role of Immunosenescence in Cerebral Small Vessel Disease: a Review. Int J Mol Sci. 2022;23(13):7136. doi:10.3390/ijms23137136
20. Danesh J, Lewington S, Thompson SG, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. J Am Med Assoc. 2005;294(14):1799–1809. doi:10.1001/jama.294.14.1799
21. Hou HQ, Xiang XL, Pan YS, et al. Baseline or 90-day fibrinogen levels and long-term outcomes after ischemic stroke or TIA: results from the China national stroke registry III. Atherosclerosis. 2021;337:35–41. doi:10.1016/j.atherosclerosis.2021.10.002
22. Wei -C-C, Zhang S-T, Liu J-F, et al. Association between Fibrinogen and Leukoaraiosis in Patients with Ischemic Stroke and Atrial Fibrillation. J Stroke Cerebrovascular Dis. 2017;26(11):2630–2637. doi:10.1016/j.jstrokecerebrovasdis.2017.06.027
23. You C-J, Liu D, Liu -L-L, Liu -Q-Q, Guo-Zhong L. Correlation between Fibrinogen and White Matter Hyperintensities among Nondiabetic Individuals with Noncardiogenic Ischemic Stroke. J Stroke Cerebrovascular Dis. 2018;27(9):2360–2366. doi:10.1016/j.jstrokecerebrovasdis.2018.04.025
24. Aribisala BS, Wiseman S, Morris Z, et al. Circulating Inflammatory Markers Are Associated With Magnetic Resonance Imaging-Visible Perivascular Spaces But Not Directly With White Matter Hyperintensities. Stroke. 2014;45(2):605–607. doi:10.1161/STROKEAHA.113.004059
25. van Oijen M, Cheung EYL, Geluk CEM, et al. Haplotypes of the fibrinogen gene and cerebral small vessel disease: the Rotterdam scan study. J Neurol Neurosurg. 2008;79(7):799–803. doi:10.1136/jnnp.2006.113035
26. Guo X, Deng B, Zhong L, et al. Fibrinogen is an Independent Risk Factor for White Matter Hyperintensities in CADASIL but not in Sporadic Cerebral Small Vessel Disease Patients. Aging Dis. 2021;12(3):801–811. doi:10.14336/AD.2020.1110
27. Zhou H, Wang A, Meng X, et al. Low serum albumin levels predict poor outcome in patients with acute ischaemic stroke or transient ischaemic attack. Stroke Vasc Neurol. 2021;6(3):458–466. doi:10.1136/svn-2020-000676
28. Belayev L, Liu Y, Zhao W, Busto R, Ginsberg MD. Human albumin therapy of acute ischemic stroke: marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke. 2001;32(2):553–560. doi:10.1161/01.str.32.2.553
29. Huang Y, Xiao Z. Albumin therapy for acute ischemic stroke: a meta-analysis. Neurol Sci. 2021;42(7):2713–2719. doi:10.1007/s10072-021-05244-9
30. Wang P, Yuan D, Zhang C, et al. High fibrinogen-to-albumin ratio with type 2 diabetes mellitus is associated with poor prognosis in patients undergoing percutaneous coronary intervention: 5-year findings from a large cohort. Cardiovasc Diabetol. 2022;21(1):46. doi:10.1186/s12933-022-01477-w
31. Hwang KT, Chung JK, Roh EY, et al. Prognostic Influence of Preoperative Fibrinogen to Albumin Ratio for Breast Cancer. J Breast Cancer. 2017;20(3):254–263. doi:10.4048/jbc.2017.20.3.254
32. Xu WY, Zhang HH, Xiong JP, et al. Prognostic significance of the fibrinogen-to-albumin ratio in gallbladder cancer patients. World J Gastroenterol. 2018;24(29):3281–3292. doi:10.3748/wjg.v24.i29.3281
33. Desai R, Fadah K, Srikanth S, Neha NFN, Jain A. Fibrinogen-albumin ratio predicting major adverse cardiovascular outcomes post-percutaneous coronary intervention: a systematic review and exploratory meta-analysis. Clin Cardiol. 2023;46(4):455–458. doi:10.1002/clc.23981
34. Zhai M, Cao S, Lu J, Xu H, Xia M, Li Z. The Relationship Between the Fibrinogen to Albumin Ratio and Early Outcomes in Patients with Acute Pontine Infarction. Clin App Thrombosis/Hemostasis. 2022;28:10760296211067260. doi:10.1177/10760296211067260
35. Zheng L, Wang Z, Liu J, et al. Association between admission blood fibrinogen-to-albumin ratio and clinical outcomes after acute lacunar stroke. Biomarker Med. 2021;15(2):87–96. doi:10.2217/bmm-2019-0537
36. Ruan Y, Yuan C, Liu Y, et al. High fibrinogen-to-albumin ratio is associated with hemorrhagic transformation in acute ischemic stroke patients. Brain Behav. 2021;11(1):e01855. doi:10.1002/brb3.1855
37. Yang M, Tang L, Bing S, Tang X. Association between fibrinogen-to-albumin ratio and hemorrhagic transformation after intravenous thrombolysis in ischemic stroke patients. Neurol Sci. 2023;44(4):1281–1288. doi:10.1007/s10072-022-06544-4
38. Markus HS, de Leeuw FE. Cerebral small vessel disease: recent advances and future directions. Int J Stroke. 2023;18(1):4–14. doi:10.1177/17474930221144911
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

