Back to Journals » Clinical Ophthalmology » Volume 18
The MOSAIC Study: A Mixed-Methods Study of the Clinical, Emotional, and Financial Burden of Geographic Atrophy Among Patients and Caregivers in the US
Authors Bakri SJ, Amoaku WM, Altman D, Quéré S, Quilantan J, Carpenter-Conlin J, Sarda SP, Jones DL, Nielsen JS
Received 22 December 2023
Accepted for publication 7 August 2024
Published 23 August 2024 Volume 2024:18 Pages 2357—2368
DOI https://doi.org/10.2147/OPTH.S455984
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Video abstract presented by Bakri.
Views: 125
Sophie J Bakri,1 Winfried MK Amoaku,2 Danielle Altman,3 Stéphane Quéré,4 Jaclyn Quilantan,5 Julia Carpenter-Conlin,5 Sujata P Sarda,5 Daniel L Jones,5 Jared S Nielsen6, †
1Department of Ophthalmology, Mayo Clinic, Rochester, MN, USA; 2Faculty of Medicine & Health Sciences, University of Nottingham, Queens Medical Centre, Nottingham, England, UK; 3Modus Outcomes, a THREAD Company, Cary, NC, USA; 4Modus Outcomes, a THREAD Company, Lyon, France; 5Apellis Pharmaceuticals, Inc, Waltham, MA, USA; 6Wolfe Eye Clinic PC, West Des Moines, IA, USA
†Dr Jared S Nielsen passed away on June 14, 2024
Correspondence: Sophie J Bakri, Department of Ophthalmology, Mayo Clinic, 200 First St. SW, Rochester, MN, 55905, USA, Tel +1 507-284-3614, Email [email protected]
Purpose: Geographic atrophy (GA) impacts both patients and caregivers, yet little is understood about their respective burdens. The MOSAIC study aimed to identify the clinical, emotional, and financial burden among patients with GA and caregivers.
Methods: A total of 28 patients with GA and 17 caregivers from the United States (US), the United Kingdom, and Australia participated in individualized qualitative interviews followed by a cross-sectional quantitative survey of 102 patients and 102 caregivers in the US. Interview transcripts were analyzed to develop conceptual models, which were then used to guide the design of quantitative surveys. Data were described at the item level and score level when appropriate (National Eye Institute Visual Function Questionnaire [NEI VFQ]-39 and Zarit Burden Interview [ZBI]). For the patient/caregiver dyad sample, the association between the NEI VFQ-39 scores and ZBI score was explored through correlation coefficients and scatterplots.
Results: GA had a substantial impact on patients’ vision-related quality of life, activities of daily living, and instrumental activities of daily living. There was considerable overlap between perspectives and key concerns identified by patients and caregivers. Eighty-three percent of caregivers reported having to drive patients to appointments due to limited patient mobility, for example, and 41% reported a change in their employment status after becoming a caregiver, with 50% of them unable to work due to caregiving. The burden of patients and caregivers had a correlation ranging from − 0.63 to − 0.21 between NEI VFQ-39 subscale and composite scores and ZBI score.
Conclusion: This study confirms the paucity of support for both patients with GA and caregivers. Both groups require expanded access to financial, social, and mental health resources.
Plain Language Summary: What is this summary about?
People with geographic atrophy, also called GA, can lose their eyesight and have a hard time driving, reading, and recognizing faces. This can worsen their quality of life. Often, people with GA need someone to care for them. The MOSAIC study was done to find out how GA affects health, happiness, and finances of people with GA and their caregivers.
What were the results?
One hundred and two people with GA and 102 caregivers in the United States were interviewed. The average age of people with GA was 68 years and of caregivers was 46 years. The findings showed that most people with GA did not drive because of their poor eyesight and instead counted on their caregivers to drive them to doctor appointments and other places. They also had a reading and doing things around their home because of their worsened eyesight.
Both people with GA and caregivers said they felt stressed. They both worried about spending money on things they need to make living with GA easier. They also felt stressed about their finances because they could not work as much. People with GA worried most about losing their independence and caregivers worried most about the future of their loved one with GA.
What do the results mean?
This study showed that GA has a serious effect on people’s health and quality of life while also having a major impact on their caregivers.
Keywords: age-related macular degeneration, functional vision, geographic atrophy, health-related quality of life, caregiver burden, disease burden
Corrigendum for this paper has been published.
Introduction
Age-related macular degeneration (AMD) is a degenerative eye disease in which deterioration of the macula causes progressive and irreversible vision loss.1 Geographic atrophy (GA) is an advanced form of AMD characterized by the progressive and irreversible loss of photoreceptors, retinal pigment epithelium, and choriocapillaris.1 Visual function deficits in reading, night vision, and dark adaptation appear gradually and often precede vision loss.1,2 Until very recently,3,4 there were no treatments available for patients with GA, and physicians could only monitor disease progression and encourage lifestyle modifications, including smoking cessation,5 adherence to a Mediterranean diet,6 vitamin supplementation,5,7 and interventions aimed at improving the ability to perform functions required for daily living, such as low vision rehabilitation.8 Pegcetacoplan and avacincaptad pegol are currently the only two therapeutics indicated for the treatment of GA secondary to AMD, approved for use in the United States (US) in 2023.3,4
GA is a leading cause of legal blindness1,9 and is responsible for substantial economic hardship for those diagnosed with the disease.10 As has been noted in prior research, general and age-related vision loss can translate to significant damage to quality of life (QoL) in ways that are often “underestimated by health status and utility measures”.2 Moreover, GA imposes a considerable burden on both people with the disease and caregivers, causing an estimated 60% decrease in the average patient’s QoL when compared to people of similar age without GA.11,12 Despite the documented negative effects of GA,13 no multi-country studies have investigated the clinical, emotional, and financial burden of the disease. Therefore, we conducted this mixed-methods study to characterize and document the impact GA has on patients and caregivers.
Methods
MOSAIC was a non-interventional study using a two-staged, sequential exploratory mixed-methods design.14 Initial qualitative interviews with patients with GA and caregivers were conducted in the US, the United Kingdom (UK), and Australia.15 Those interviews informed the design of a cross-sectional quantitative survey that was administered to patients with GA and caregivers in the US, Canada, Australia, the UK, Germany, and France a few months after the completion of the qualitative interviews.15 The quantitative results reported herein include only the US data because this was the largest single-country cohort that participated in the MOSAIC study.
Patients were invited to take part in the study if they were aged ≥60 years, self-reported a diagnosis of GA secondary to AMD in at least one eye, did not have neovascular AMD, and had GA that could not be due to Stargardt disease. In addition, after data review, patients who reported being diagnosed with GA before 50 years of age were excluded to mitigate against potentially misstated diagnoses by participants (9 patients were excluded from the US sample for this reason). The study design involved convenience sampling, which is standard practice for burden of illness studies.16–18 Caregivers were invited to participate in the study if they were aged ≥18 years, were the primary caregiver of a patient with GA, and received no financial compensation for their caregiving services. A primary caregiver was defined as any person who, without being a professional or belonging to a social support network, was in some way directly involved in the patient’s care or was directly affected by the patient’s health condition. Patient advocacy groups, including the American Macular Degeneration Foundation, BrightFocus Foundation, MD Support, Prevent Blindness, and SupportSight Foundation, supported recruitment and screening of potential US participants. Further recruitment was supported by Global Perspectives, a recruitment agency.
Patients and caregivers were interviewed and surveyed separately, and while patient/caregiver dyads (ie, paired participants with an established patient/caregiver relationship) were desirable, not all patients or caregivers were associated with another participant in the study. The survey was mostly administered electronically, but patients who were too vision-impaired to take an online survey on their own had the ability to have the survey moderated by phone by a Global Perspectives interviewer. Study documents, including the protocol, patient demographic and health information forms, interview guides, surveys, screeners, and informed consent forms received ethical approval from an independent board, Solutions IRB (IRB# IORG0007116). All participants provided informed consent to participate. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments.
Qualitative Methods
Conduct of Interviews
Eligible participants were purposively sampled and took part in individualized interviews conducted online or by phone in English. Interviews lasted approximately 60 minutes and were audio-recorded. Participants provided verbal permission to audio record the interview for subsequent transcription. Interviews were transcribed verbatim and anonymized for participant privacy. Interviews followed a semi-structured interview guide, one for patients with GA and one for caregivers. Patient interviews were focused on changes in vision due to GA, the impacts of living with GA, and how patients manage GA (eg, medical appointments). Caregiver interviews were focused on understanding the types of support the caregivers provide to patients and the impacts of caregiving on their own life.
Qualitative Coding and Analysis
Transcripts were generated by Fantastic Transcripts, an independent transcription agency, reviewed by transcription agency senior staff, then reviewed again by study personnel prior to coding. Transcripts were coded and analyzed with thematic analysis in ATLAS.ti Scientific Software Development GmbH (2023) ATLAS.ti MAC (version 9 [computer program] available at https//atlasti.com, downloaded May 2, 2021) using an inductive approach tailored to the study objectives.19 Prior to coding, a list of provisional codes was generated based on the major interview guide topics (eg, visual changes); provisional codes were further specified during coding (eg, visual changes: blurred vision). The first transcript was independently coded by two researchers (DA and TL); any inconsistencies in codes were discussed and reconciled. An initial coding framework was developed, which was revised throughout the study as needed.
Following coding, conceptual models were developed by one researcher (DA) in the form of a visual representation of the clinical, emotional, and financial burden on patients with GA (Figure 1a) and caregivers (Figure 1b). A second researcher (TL) confirmed the accuracy of the conceptual models by reviewing the models against the final coding framework in ATLAS.ti. The conceptual models were developed using standard analytical techniques.20,21 Codes, derived from patient and caregiver quotations, were inductively categorized into higher-order overarching categories including domains and sub-domains, reflecting their conceptual underpinning. In addition, the adequacy of the sample size was assessed by a saturation analysis performed to determine how much new information about the patient or caregiver experience of GA was obtained in each group. Saturation analysis was conducted separately on patient and caregiver transcripts at the subdomain level with patient and caregiver data combined on GA-related symptoms and impacts.
Quantitative Methods
The analyses were performed in three analysis sets: the patient population, the caregiver population, and the patient/caregiver dyad population. A descriptive analysis was conducted for each set: socio-demographic and health variables were described along with patient-reported outcome data at the item level and at the score level for the National Eye Institute Visual Function Questionnaire (NEI VFQ)-39 and Zarit Burden Interview (ZBI) total score. The NEI VFQ-39 is used to measure patient-reported vision-dependent function and the impact of vision loss on health-related QoL.22 The NEI VFQ-39 consists of the same 12 subscales as the NEI VFQ-25, including subscales specific to driving, vision difficulty, and mental health, with optional subscale-specific questions that can be added to improve the reliability of the subscale being assessed.23 The subscale and composite NEI VFQ-39 scores range from 0 to 100, with higher scores indicating better QoL. For the caregiver population, caregiver-reported outcome data were reported at the item level and at the score level for the ZBI total score. To better interpret the ZBI total score, four categories were defined: (1) “little or no burden” (score: 0–20), (2) “mild to moderate burden” (score: 21–40), (3) “moderate to severe burden” (score: 41–60), and (4) “severe burden” (score: 61–88). The patient NEI VFQ-39 score was described according to caregiver ZBI total score using the four categories provided above. The association between the ZBI total score and the NEI VFQ-39 subscale and composite scores was explored through correlation coefficients and scatterplots. A statistical analysis plan was finalized before the availability of the final data and analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA).
Results
Qualitative Analysis Results
A total of 28 patients (22 US residents, four UK residents, and two Australian residents) and 17 caregivers (11 US residents, four UK residents, and two Australian residents) participated in qualitative interviews. A conceptual model derived from patient and caregiver interview data documents the clinical, emotional, and financial burden of GA. Results of the interviews performed with the patients with GA and caregivers revealed significant impact of visual changes on patients (Figure 1a) and caregivers (Figure 1b). These qualitative findings helped determine the nature of the quantitative study instruments.
Quantitative Analysis Results
Socio-Demographic and Clinical Characteristics of Patient and Caregiver Samples
The patient sample was composed of 102 patients residing in the US. The majority of patients were female (57%). The mean (standard deviation [SD]) age was 68 (4) years and ranged from 60 to 84 years. The sample was largely White (68%). Most of the patients were retired (77%), and 88% of patients reported living with someone (partner or spouse, family member, or friends). Nearly all patients had a level of education at least equivalent to high school graduation (Table 1).
 |
Table 1 Patient and Caregiver Socio-Demographics. GED, General Educational Development; NA, Not Applicable |
The caregiver sample comprised 102 caregivers, the majority of whom were White (62%), with a similar proportion of male (47%) and female (53%) caregivers. The mean (SD) age was 46 (15) years and ranged from 19 to 68 years. All caregivers had a level of education at least equivalent to high school graduation. A total of 42% of caregivers reported being employed; 21% were working full-time and 22% were working part-time. Among all patients, 34% were not employed, 12% reported being homemakers, and 11% were retired. Caregivers reported having provided support for a mean (SD) of 3.3 (4.7) years to the patient with GA they cared for, and 81% of caregivers reported living with the person they cared for.
A total of 75% of patients reported experiencing visual changes: 43% experienced changes in both eyes and 32% experienced visual changes in only one eye. The mean (SD) age at time of first experience of visual changes due to GA and the mean (SD) age at time of GA diagnosis were similar at 64 (5) years (range: 51–82) and 62 (6) years (range: 50–82), respectively (Table 2).
 |
Table 2 GA and Other Eye Conditions Experienced by Patients. AMD, Age-Related Macular Degeneration; GA, Geographic Atrophy; SD, Standard Deviation |
Impact of GA on Patients’ Activities of Daily Living (ADLs) and Instrumental Activities of Daily Living (IADLs) and Caregiver Burden
GA impacted patients’ daily activities by limiting their mobility as well as their ability to accomplish daily activities independently. Each of these limitations in turn had ramifying effects on caregivers.
Nearly 86% of patients (n = 88) stated that they did not currently drive. Among the 84 patients who previously drove, 76 stated that they were no longer driving because of their eyesight only or both eyesight and other reasons. For those who still drove (14%, n = 14), driving difficulty increased at night and in challenging conditions such as in bad weather, during rush hour, on the freeway, or in city traffic. The mean (SD) driving subscale score of the NEI VFQ-39, 9.7 (20.4), was the lowest score compared with the other subscales, indicating a negative impact of GA on driving. Over half of the patients (51%) reported they could walk independently in familiar places, but only 22% reported being able to walk independently in unfamiliar places. Patients also reported that GA impacted their ability to independently accomplish activities within the home, including heavy-duty house cleaning (65%), preparing complex meals (64%), minor house repairs (59%), and locating an object that is not in its usual place (55%) (Figure 2). Moderate to extreme difficulty reading a newspaper and small print (eg, medicine bottle, legal forms) was reported by 67% and 68% of patients, respectively. The proportions of patients who reported no longer reading the newspaper and small print were 19% and 20%, respectively. Reading street signs was also reported to be of moderate to extreme difficulty for 61% of patients, with 22% of patients reporting that they no longer read street signs because of their eyesight. The use of extra lighting and increased font size to assist with reading was reported by 64% and 84% of patients, respectively.
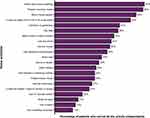 |
Figure 2 Home activities and the proportion of patients who report being unable to complete them independentlya. Abbreviation: TV, television. Note: aN = 102. |
Limitations on patient mobility outside the home had a direct impact on caregiver burden. Eighty-three percent (83%) of caregivers reported having to drive patients to their eye appointments for GA and 76% reported having to drive patients to other medical appointments. Notably, 23% of caregivers described driving the person they cared for as the most inconvenient form of care given. In addition, 74% of caregivers reported shopping for the person they cared for and 72% of caregivers reported accompanying the person they cared for while they were walking. Caregivers corroborated patients’ reports of how GA impacted their ability to read and perform activities within the home. Caregivers reported spending a median time of 10 (interquartile range: 5–25) hours per week on household tasks that the patients would have been able to perform independently if not for their visual impairment due to GA. Assisting in managing medications, including reading medicine bottles, was reported by 62% of caregivers.
Financial Impact on Patients and Caregivers Due to GA
Patients and caregivers also reported significant financial burden, with 44% of patients and 37% of caregivers being concerned or very concerned about the effect of GA on their finances in the future. Patients and caregivers reported using their own money to purchase products to assist the patient with GA, including items such as extra lighting (63% and 52%, respectively), eyeglasses (72% and 51%, respectively) or devices to assist with reading (64% and 50%, respectively). Among patients with GA, 37% (n = 38) reported that GA had affected their ability to do paid work and, of those, 61% had to stop working or retired sooner than they wanted.
Impact on work was even more significant for caregivers than for patients, likely because of the younger age of the caregiver cohort. A relatively high proportion of caregivers (41%) had a change in their employment status after becoming a caregiver, with 50% of them unable to work due to caregiving; 29% reported working fewer hours, and 12% retired early.
Emotional Impact on Patients and Caregivers Due to GA
GA also impacted patients’ and caregivers’ psychological and emotional wellbeing. Losing their independence was the biggest concern reported by 56% of patients; 51% of patients worried about their eyesight most or all the time. Patients reported feeling frustrated a lot of the time (47%), and 67% reported feeling much less control over what they did (answers on the NEI VFQ-39 questionnaire of “definitely true” or “mostly true”). About 34% of patients reported worrying about doing things that would embarrass themselves or others because of their eyesight and 28% reported being often irritable. Mean (SD) vision-specific mental health subscale score, related to the items described previously, was 46.4 (25.1).
Regarding caregivers, mean (SD) ZBI total score was 24.8 (18.7) on a scale ranging from 0 to 88, in which higher score indicates more severe burden. Most caregivers (82%) reported being afraid of what the future held for the person they cared for (item 7) and 64% reported feeling the person they cared for was dependent on them (item 8) “sometimes”, “quite frequently”, or “nearly always”.
Despite these concerns, 62% of caregivers indicated that providing care gave their life meaning “to a great extent” and 53% reported that providing support to the person they cared for improved their relationship with them “to a great extent”.
Caregiver Burden and Vision-Related QoL: Evidence from the US Dyad Sample
Among caregivers participating in the survey, 93 were related to a patient who also participated. Analyzing relationships between patient and caregiver responses provided insights regarding the ways in which patient and caregiver burden were similar. Overall, Spearman rank-order correlation coefficients between NEI VFQ-39 subscale and composite scores and ZBI total score ranged from −0.63 to −0.21, indicating a correlation between vision-related QoL of the patient and the caregiver’s burden.
There was a clear association between poor vision-related QoL of patients with GA and high caregiver burden (Figure 3). Mean NEI VFQ-39 composite score was lower, indicating poorer vision-related QoL, for the subgroup of patients whose caregiver had a higher ZBI total score, which indicated more burden (mean values of 15.8, 26.5, 35.9, and 53.0 for patients whose caregivers reported “severe burden”, “moderate to severe burden”, “mild to moderate burden”, and “little or no burden”, respectively). The mean NEI VFQ-39 General health subscale score, corresponding to the single item asking patients about their general health, was higher for patients receiving help from caregivers reporting lower burden (mean values of 16.3, 25.0, 33.1, and 49.0 for patients whose caregivers reported “severe burden”, “moderate to severe burden”, “mild to moderate burden”, and “little or no burden”, respectively). In terms of mental health, caregivers reporting higher burden took care of patients who reported having lower mental health subscale score, indicating more emotional impact due to GA (mean values of 26.3, 35.9, 36.3, and 57.5 for patients whose caregivers reported “severe burden”, “moderate to severe burden”, “mild to moderate burden” and “little or no burden”, respectively).
Discussion
The results of the MOSAIC study support that patients with GA and caregivers experience a high degree of clinical, emotional, and financial burden related to GA, with considerable overlap noted between perspectives and key concerns identified by both patients with GA and caregivers. Vision loss due to GA reduced patients’ ability to function safely and independently outside and inside the home. A negative impact on driving and the ability to perform household tasks independently were among the most frequent challenges reported by patients with GA and caregivers. Loss of autonomy and independence reported by patients were found to be the greatest concerns affecting patient emotional well-being. Caregiver emotional well-being was primarily affected by concern of the future for the person they cared for and how much care they needed to provide. The financial burden experienced by patients with GA and caregivers was related to needing to spend personal money for medical care and assistive devices, the need to change employment status negatively impacting earning potential, and worry about future financial issues. Caregiver employment status was more often adversely affected compared with patient employment status, likely due in part to the younger age of the caregiver cohort. For patients, lower NEI VFQ-39 scores were correlated with higher GA-related impacts on QoL, meaning that these patients were more likely to need support from caregivers. Caregivers with higher ZBI scores reported a more significant burden from their caretaking activities, suggesting that these caregivers were also greatly impacted by GA. Patient vision-related QoL and caregiver burden were correlated when assessing the association between the NEI VFQ-39 subscale and composite scores and the ZBI total score in the patient/caregiver dyad population.
The study findings indicate that GA may adversely affect patients’ ability to perform ADLs and IADLs independently. ADLs include the ability to ambulate and feed oneself independently. IADLs include the ability to drive, shop, prepare meals, and perform house cleaning and home maintenance independently.24 Similar to other studies,10,11 the patients in this study reported that GA had impacted their ability to drive. Caregivers corroborated this finding, reporting the need to drive patients to appointments, with almost a quarter of caregivers reporting this to be the most inconvenient form of care they provided. Patients in this study also reported difficulty in walking independently in unfamiliar places, preparing complex meals, and performing heavy-duty house cleaning and minor house repairs. These findings were also supported by caregiver report of needing to shop for the person they care for and accompany them while walking, spending 10 hours per week performing household tasks that the patient could no longer perform independently, and assisting the patient with reading activities, including medication management.
The findings of this study also support the urgent need for access to treatment and broader resources and support to help mitigate the clinical, financial, and humanistic burden of GA. Currently, only two treatments are approved in the US for the treatment of GA secondary to AMD.3,4 Continued development of therapies to treat GA and referral to low vision rehabilitation and occupational therapy may assist patients and caregivers alike. Use of therapeutics has been shown to slow GA progression.25,26 Referral for low vision rehabilitation and occupational therapy can assist patients and caregivers through education on the use of assistive devices/aids and other adaptations for patients to be able to perform everyday tasks and chores independently or with minimal assistance.8,27 From a public policy perspective, ensuring assistive devices and healthcare interventions are covered by insurance including Medicare is critical to alleviating the financial burden associated with GA.10 Better social support systems may lessen feelings of isolation and other aspects of the emotional burden of GA that patients face.27,28 Patient advocacy groups can help connect patients with GA and caregivers to others living with GA and improve patient and caregiver understanding of the range of technologies available that may improve vision-related QoL for patients and lessen caregiver burden (eg, magnifiers, color contrast chopping boards, text-to-speech software).27,28
While MOSAIC provides a rich data set on the impact of GA on the lives of patients and caregivers, there are some limitations to the study. First, the study relied on patient self-reporting of diagnoses and visual changes attributed to GA, not confirmed by clinicians. Second, the NEI VFQ-39 was utilized to measure visual function. The NEI VFQ-39 is a modification of the NEI VFQ-25, which was developed to measure a range of general vision disorders.22 While the NEI VFQ-25 is commonly used in GA studies and has evidence to support its use as a reliable and valid measure of patient visual function and vision-related QoL in the GA population, it has not been specifically validated in the GA population.29–31 Correlations and interdependencies between factors in the conceptual models developed for patients with GA and caregivers were not performed. Finally, the study utilized convenience sampling. Convenience sampling, in combination with the potential weaknesses related to the relevancy and understandability of the NEI VFQ-39 in the GA population, limit the generalizability of the study findings.16,18,32
Further research, such as cognitive interviews with patients, is needed to explore the understandability and relevancy of the NEI VFQ-39 to patients with GA. Psychometric research to explore the reliability and construct validity of the NEI VFQ-39 in the GA population is also needed. Novel items or additional patient-reported outcome questionnaires may be required in GA clinical trials to ensure the comprehensive measurement of vision-related QoL. Future studies to consider for building on the findings of this study are needed to assess whether any feedback loops between patients with GA and caregivers exist, such as the impact the emotional burden of the caregiver has on the care provided to the patient with GA. Studies on the use of low vision rehabilitation and healthcare resource utilization associated with GA are also recommended to better determine the impact of low vision rehabilitation on improving independence and QoL for patients with GA and to better understand the global incremental cost of GA, including patients with early or intermediate AMD.33 Understanding the current cost of care for patients with early or intermediate AMD would provide a baseline for future studies to assess the financial impact of early initiation of treatment with therapeutics and low vision rehabilitation.31
Conclusion
The results from this study provide evidence that GA significantly impacts both patients and caregivers, whose perspectives overlap to a striking degree. This research also indicates that both patients with GA and caregivers are substantially underserved and require increased access to disease-specific resources, particularly social and mental health support. Available treatment options for patients, including access to low vision rehabilitation and GA-specific therapies, remain an unmet need that, once addressed, may help improve patient independence and QoL and decrease caregiver burden. The data from this study provide a strong foundation for future studies to build upon when investigating patient and caregiver experiences with GA.
Prior Presentation
Results from the qualitative portion of the analysis were previously presented at the 2022 ARVO Annual Meeting, Denver, CO, May 1–4, 2022, and virtually. The poster’s abstract was published in “2022 Abstract Issue” in Invest Ophthalmol Vis Sci (A0145).
Data Sharing Statement
The data reported are available from the sponsor upon reasonable request. Individual participant data will not be made available. Requests for access to data should be addressed to [email protected]. The study protocol will be available with no end date. All proposals requesting data access will need to specify how the data will be used.
Acknowledgments
Several patient advocacy groups supported recruitment and screening of potential US participants, feedback on study design, and interpretation of results, including Matthew Levine from the American Macular Degeneration Foundation, Diana Campbell from BrightFocus Foundation, Dan Roberts from MD Support, Kira Baldonado from Prevent Blindness, and Dawn Prall from SupportSight Foundation. Diana de la Puente and Eva García from Global Perspectives contributed to participant recruitment, screening, and survey administration. Editorial support was provided by Trais Pearson, PhD (Modus Outcomes), Lori Bacarella (an employee of Modus Outcomes at time of her involvement), and OPEN Health Communications, and funded by Apellis Pharmaceuticals. Teya Lovell (an employee of Modus Outcomes at time of her involvement) provided qualitative coding support. Deborah Bouvier, PhD, and Roy Schwartz, MD, PhD, of Apellis Pharmaceuticals provided editorial assistance.
This paper is dedicated to the memory of Dr. Jared S. Nielsen who devoted his career to his patients and their vision.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This research was funded by Apellis Pharmaceuticals.
Disclosure
Sophie J. Bakri reports consulting fees from AbbVie, Adverum, Amgen, Annexon, Apellis, Aviceda, Cholgene, Eyepoint, iLumen, Iveric Bio, Genentech, Kala, Neurotech, Novartis, Pixium, Ocular Therapeutix, Outlook Therapeutics, Regeneron, Rejuvitas, Regenxbio, Roche, VoxelCloud, and Zeiss; and stock or options in Revana. Winfried M. K. Amoaku reports grants from Bayer and Boehringer Ingelheim; consulting fees from AbbVie, Alimera, Allergan, Apellis, Novartis, and Roche; speaking fees from AbbVie, Novartis, and Roche; serving on an advisory board for Bioeq; and being an unpaid board member for the Convention for Biomedical Research, Ghana. Danielle Altman was an employee of Modus Outcomes at the time of the study. Stéphane Quéré is an employee of Modus Outcomes, which was contracted by Apellis Pharmaceuticals on this study. Jaclyn Quilantan and Sujata P. Sarda are employees of Apellis Pharmaceuticals. Julia Carpenter-Conlin was an employee of Apellis Pharmaceuticals at the time of the study. Daniel L. Jones is an employee of Apellis Pharmaceuticals and has equity interest in the company. Jared S. Nielsen (deceased) reported consulting fees from Astellas, Apellis, Gyroscope, Genentech Roche, Regeneron, Iveric Bio, Novartis, and Kodiak Scientific; and served on advisory boards for Genentech, Iveric Bio, and Regeneron. He also participated in clinical trials for Gemini Therapeutics, Clearside, Regeneron, Ocular Therapeutics, Regenxbio, Bayer, and Novo Nordisk. The authors report no other conflicts of interest in this work.
References
1. Holz FG, Strauss EC, Schmitz-Valckenberg S, van Lookeren Campagne M. Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology. 2014;121(5):1079–1091. doi:10.1016/j.ophtha.2013.11.023
2. Mitchell J, Bradley C. Quality of life in age-related macular degeneration: a review of the literature. Health Qual Life Outcomes. 2006;4(1):97. doi:10.1186/1477-7525-4-97
3. Kang C. Avacincaptad pegol: first approval. Drugs. 2023;83(15):1447–1453. doi:10.1007/s40265-023-01948-8
4. Nadeem A, Malik IA, Shariq F, et al. Advancements in the treatment of geographic atrophy: focus on pegcetacoplan in age-related macular degeneration. Ann Med Surg Lond. 2023;85(12):6067–6077. doi:10.1097/MS9.0000000000001466
5. Thomas CJ, Mirza RG, Gill MK. Age-related macular degeneration. Med Clin North Am. 2021;105(3):473–491. doi:10.1016/j.mcna.2021.01.003
6. Agrón E, Mares J, Chew EY, Keenan TDL. Adherence to a Mediterranean diet and geographic atrophy enlargement rate: Age-Related Eye Disease Study 2 report 29. Ophthalmol Retina. 2022;6(9):762–770. doi:10.1016/j.oret.2022.03.022
7. Agrón E, Mares J, Clemons TE, Swaroop A, Chew EY, Keenan TDL. Dietary nutrient intake and progression to late age-related macular degeneration in the Age-Related Eye Disease Studies 1 and 2. Ophthalmology. 2021;128(3):425–442. doi:10.1016/j.ophtha.2020.08.018
8. Erginturk Acar D, Batioglu F, Idil A, Sahli E, Goksuluk D. Rehabilitation methods for patients with geographic atrophy due to age-related macular degeneration and effects of rehabilitation on quality of life. J Ophthalmol. 2023;2023(1):3389750. doi:10.1155/2023/3389750
9. Rees A, Zekite A, Bunce C, Patel PJ. How many people in England and Wales are registered partially sighted or blind because of age-related macular degeneration? Eye (Lond). 2014;28(7):832–837. doi:10.1038/eye.2014.103
10. Sarda SP, Heyes A, Bektas M, et al. Humanistic and economic burden of geographic atrophy: a systematic literature review. Clin Ophthalmol. 2021;15:4629–4644. doi:10.2147/OPTH.S338253
11. Patel PJ, Ziemssen F, Ng E, et al. Burden of illness in geographic atrophy: a study of vision-related quality of life and health care resource use. Clin Ophthalmol. 2020;14:15–28. doi:10.2147/OPTH.S226425
12. Brown GC, Brown MM, Sharma S, et al. The burden of age-related macular degeneration: a value-based medicine analysis. Trans Am Ophthalmol Soc. 2005;103:173–184.
13. Jones D, Nielsen J, Altman D, et al. MOSAIC: a qualitative study of the clinical, humanistic, and financial burden of geographic atrophy (GA) among patients [abstract]. Invest Ophthalmol Vis Sci. 2022;63(7):A0145.
14. Creswell JW, Plano Clark VL. Designing and Conducting Mixed Methods Research.
15. Amoaku W, Bakri S, Altman D, et al. A mixed-methods study of the clinical, humanistic, and economic burden of geographic atrophy (GA) among patients and their unpaid caregivers [abstract]. Presented at: EURETINA, Hamburg, Germany.
16. Dingli D, Matos JE, Lehrhaupt K, et al. The burden of illness in patients with paroxysmal nocturnal hemoglobinuria receiving treatment with the C5-inhibitors eculizumab or ravulizumab: results from a US patient survey. Ann Hematol. 2022;101(2):251–263. doi:10.1007/s00277-021-04715-5
17. Panse J, Sicre de Fontbrune F, Burmester P, et al. The burden of illness of patients with paroxysmal nocturnal haemoglobinuria receiving C5 inhibitors in France, Germany and the United Kingdom: patient-reported insights on symptoms and quality of life. Eur J Haematol. 2022;109(4):351–363. doi:10.1111/ejh.13816
18. de Fontbrune FS, Burmester P, Piggin M, et al. The burden of illness of patients with paroxysmal nocturnal haemoglobinuria receiving C5 inhibitors: clinical outcomes and medical encounters from the patient perspective. Hematology. 2022;27(1):1140–1151. doi:10.1080/16078454.2022.2127630
19. Naeem M, Ozuem W, Howell K, Ranfagni S. A step-by-step process of thematic analysis to develop a conceptual model in qualitative research. Int J Qual Methods. 2023;22:1–18. doi:10.1177/16094069231205789
20. Bowling A. Research Methods in Health: Investigating Health and Health Services.
21. Bryman A, Burgess B. Analyzing Qualitative Data. Routledge; 2002.
22. Nassiri N, Mehravaran S, Nouri-Mahdavi K, Coleman AL. National Eye Institute Visual Function Questionnaire: usefulness in glaucoma. Optom Vis Sci. 2013;90(8):745–753. doi:10.1097/OPX.0000000000000003
23. Mangione CM The National Eye Institute 25-Item Visual Function Questionnaire (VFQ-25). 2000. Available from: https://www.nei.nih.gov/learn-about-eye-health/outreach-resources/outreach-materials/visual-function-questionnaire-25.
24. Edemekong PF, Bomgaars DL, Sukumaran S, Schoo C. Activities of Daily Living. Treasure Island, FL, USA: StatPearls Publishing LLC; 2024.
25. Jaffe GJ, Westby K, Csaky KG, et al. C5 inhibitor avacincaptad pegol for geographic atrophy due to age-related macular degeneration: a randomized pivotal Phase 2/3 trial. Ophthalmology. 2021;128(4):576–586. doi:10.1016/j.ophtha.2020.08.027
26. Heier JS, Lad EM, Holz FG, et al. Pegcetacoplan for the treatment of geographic atrophy secondary to age-related macular degeneration (OAKS and DERBY): two multicentre, randomised, double-masked, sham-controlled, Phase 3 trials. Lancet. 2023;402(10411):1434–1448. doi:10.1016/S0140-6736(23)01520-9
27. Carlton J, Barnes S, Haywood A. Patient perspectives in geographic atrophy (GA): exploratory qualitative research to understand the impact of GA for patients and their families. Br Ir Orthopt J. 2019;15(1):133–141. doi:10.22599/bioj.137
28. Caswell D, Caswell W, Carlton J. Seeing beyond anatomy: quality of life with geographic atrophy. Ophthalmol Ther. 2021;10(3):367–382. doi:10.1007/s40123-021-00352-3
29. Sivaprasad S, Tschosik E, Kapre A, et al. Reliability and construct validity of the NEI VFQ-25 in a subset of patients with geographic atrophy from the phase 2 Mahalo study. Am J Ophthalmol. 2018;190:1–8. doi:10.1016/j.ajo.2018.03.006
30. Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-item National Eye Institute Visual Function Questionnaire. Arch Ophthalmol. 2001;119(7):1050–1058. doi:10.1001/archopht.119.7.1050
31. Burguera-Giménez N, García-Lázaro S, España-Gregori E, et al. Multimodal evaluation of visual function in geographic atrophy versus normal eyes. Clin Ophthalmol. 2020;14:1533–1545. doi:10.2147/OPTH.S246245
32. Jager J, Putnick DL, Bornstein MH. More than just convenient: the scientific merits of homogeneous convenience samples. Monogr Soc Res Child Dev. 2017;82(2):13–30. doi:10.1111/mono.12296
33. Krogh Nielsen M, Hinnerskov JMV, Sørensen TL. Geographic atrophy—signs, symptoms, and quality of life. Acta Ophthalmol. 2023;101(8):896–902. doi:10.1111/aos.15794
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



