Back to Journals » Clinical Ophthalmology » Volume 18
The Multifocal Pathway: A Pilot Study of a Trainee-Led Multifocal Intraocular Lens Protocol in a Tertiary Referral Hospital in Australia
Authors Sartor L, Go CZQ, Kong CF, Yeung STW, White A, Samarawickrama C
Received 30 June 2024
Accepted for publication 30 October 2024
Published 11 December 2024 Volume 2024:18 Pages 3693—3706
DOI https://doi.org/10.2147/OPTH.S484884
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Lauren Sartor,1– 3,* Christopher Ze Qian Go,1,4,* Cheng F Kong,1,* Season TW Yeung,1 Andrew White,1,2,4 Chameen Samarawickrama1– 3
1Department of Ophthalmology, Westmead Hospital, Sydney, NSW, Australia; 2Translational Ophthalmic Research and Immunology Consortium, Westmead Institute for Medical Research, Sydney, NSW, Australia; 3Department of Medicine and Health, University of Sydney, Sydney, NSW, Australia; 4Save Sight Institute, University of Sydney, Sydney, NSW, Australia
*These authors contributed equally to this work
Correspondence: Chameen Samarawickrama, Translational Ocular Research and Immunology Consortium (TORIC), Westmead Institute for Medical Research, 176 Hawkesbury Road, Westmead, NSW, 2145, Australia, Email [email protected]
Purpose: To develop a selection pathway to facilitate the use of multifocal intraocular lenses (mfIOLs) in cataract surgery in a public hospital setting.
Methods: A single-surgeon prospective cohort study in an Australian tertiary referral public hospital was conducted. A mfIOL selection pathway was designed and assessed. Outcomes measured included unaided distance (UDVA), intermediate (UIVA) and near visual acuity (UNVA), dysphotopsia, spectacle dependence and satisfaction. Patient-reported outcome measures (PROMs) were assessed using Catquest-9SF (CQ) and Near Visual Acuity Questionnaire (NAVQ). A cost-analysis was performed.
Results: Fifty-four eyes from 27 patients underwent cataract surgery with mfIOL implantation. The monocular UDVA (mean ± standard deviation) was 0.05 ± 0.12 logMAR; UIVA 0.19 ± 0.05 logMAR; UNVA 0.28 ± 0.14 logMAR; 87% and 98% of eyes achieved within 0.5D and 1.0D of target refraction respectively. Spectacle independence was 85% at distance, 81% at intermediate, 59% at near vision. High satisfaction was reported with CQ (> 85%) and NAVQ (100%). The cost difference between bilateral monofocal and mfIOLs is comparable to a pair of spectacles. Projected annual cost to the health system for a 5%– 10% eligibility rate is 1.1– 2.3 million Australian dollars.
Conclusion: The selection pathway presented overcomes the challenges in patient selection inherent to a public hospital setting and was implemented by a senior trainee with excellent vision and PROMs. The pathway ensures the cost-effectiveness of mfOL implantation. There are several funding models that can be applied to support equitable access and improved visual outcomes with mfIOLs within the government funded health system.
Plain Language Summary: Cataract surgery is a safe and effective procedure that is performed in both the private and public sectors. Traditional intraocular lenses offer clear vision at one distance, meaning that spectacles are required post-surgery. Advances in lens technology now offer the possibility of multifocality, that is, clear vision at two or more distances and the possibility to remain spectacle free. These multifocal intraocular lenses (mfIOLs) are not readily available in the public sector, due to the complexity of patient selection and of trainee experience with the mfIOLs.
In this study, conducted at Westmead Hospital in Sydney, Australia, researchers aimed to develop a pathway for patient selection for mfIOLs. The study evaluated outcomes including the resultant visual acuity, the experience of visual disturbances, dependence on glasses and patient satisfaction. They also performed a cost analysis.
The results showed that the pathway was successful and that most patients achieved excellent visual outcomes with mfIOLs, with high satisfaction rates reported. Around 85% were able to see well without glasses at a distance, 81% at intermediate distances, and 59% at near distances. The additional cost of mfIOLs was found to be comparable to the cost of glasses over time, making them a cost-effective option.
In conclusion, the study demonstrated that the selection pathway effectively addressed challenges in choosing patients for mfIOLs in public hospitals, which can facilitate access to mfIOLs for public patients.
Keywords: multifocal IOL, selection pathway, visual outcomes, satisfaction, cost analysis
Introduction
Cataract surgery is one of the most common elective procedures performed internationally1–3 and is a safe procedure with only a 0.8%–6.3% rate of any intraoperative complication.4–6 Advancements in intraocular lens (IOL) technology over the past 35 years has led to a wide selection of IOLs that offer more than the standard monofocal distance vision correction. Bi- and tri-focal IOLs offer the possibility of spectacle independence following surgery, thereby improving quality of life and convenience for patients.7,8 This has led to an increased use of mfIOLs, particularly as technological advances have resulted in satisfactory and perhaps more importantly, reliable visual outcomes.9
To achieve the visual outcomes promised by mfIOLs, refractive accuracy is critical, as even small deviations from the emmetropic target are associated with a rapid deterioration in vision.8,10 For this reason, among many others, mfIOLs are not routinely used in training hospitals. Limitations that increase variability include shared care by a team of trainees through the pre-operative, operative and post-operative appointments, variations in the quality of biometry acquisition, limitations on consult time in a high-volume setting, and trainee inexperience from patient selection and counselling through to implantation of the IOLs. Despite these limitations, if good outcomes can be achieved, the introduction of mfIOLs in a public hospital setting is advantageous to both patient and trainee. From a patient perspective, it allows access to technology and visual outcomes that can improve their quality of vision and quality of life.11–13 From a trainee perspective, it allows an opportunity to develop the knowledge and technical skills in an area that is increasingly expected by patients. However, it is critical that good outcomes be demonstrated first to justify the increased cost associated with these premium IOLs.
This study aimed to determine if mfIOLs can be successfully implanted in the highly variable training environment of a tertiary referral public hospital by a trainee surgeon. A protocol was created for trainees who had no prior experience with mfIOLs to aid their decision-making in selecting appropriate patients for mfIOL implantation. Beyond clinical outcomes across distance, intermediate and near, we collected patient reported outcome measures (PROMs) to determine the patient experience and conducted a cost analysis to determine the value in using these premium IOLs. The overarching goal was to determine if it is feasible to use mfIOLs in a teaching hospital with trainee-led assessments, and to develop a protocolised system to overcome the limitations inherent in a large teaching hospital setting.
Method
This study was conducted at Westmead Hospital, Sydney, Australia. The project was approved by the Western Sydney Local Health District Human Research Ethics Committee (HREC 2019–10) and followed the tenets of the Declaration of Helsinki.
Clinical Setting
The study was conducted in the Outpatient Ophthalmology Department of Westmead Hospital. The department is staffed by 5–6 trainees with varying levels of clinical knowledge and skills. Every three months, a new allocation of trainees is provided. The cataract assessment clinic typically accommodates 25–35 new referrals per 4-hour session and are overseen by a consultant surgeon or senior trainee alongside a junior trainee. Patient flow through the clinic includes sign-in, screening by nursing staff including visual acuity and intraocular pressure. The patient is then assessed for cataract surgery by the trainee or surgeon. Additional scans including OCT, A-scan or Pentacam are ordered at subsequent allied health appointments and are not typically overseen by medical staff. The high-volume nature and flow of the clinic, the lack of screening protocols and the variability in trainee experience with the mfIOLs are all limitations to their use in this setting.
Participant Selection
Eligible participants over 18 years of age with visually significant bilateral cataracts, an expected postoperative visual outcome of logMAR <0.1, and a strong desire for spectacle independence were selected. Exclusion criteria for the study included previous intraocular or corneal surgery, irregular corneal astigmatism, axial length less than 23 mm or greater than 25 mm, or comorbid ocular pathology that could affect visual acuity (eg maculopathy, glaucoma, diabetic retinopathy).
Participants were recruited from the general cataract assessment clinics and internally referred directly to the senior operating trainee surgeon (CG). During the consultation with the trainee, information describing the IOL technology was given to patients, and they were counselled on the risks, benefits and side effect profile of mfIOLs. Participants were given the opportunity to seek additional counselling before consenting and were able to withdraw at any time. Multifocal IOLs available for implantation were the Tecnis Synergy (Johnson & Johnson Vision, California, USA), Lentis Mplus MF30 (Teleon, Berlin, Germany) and AT LISA Tri (Carl Zeiss Meditec, Jena, Germany), and a random number generator was used to allocate patients. Outcomes from all mfIOL were pooled for analysis as the aim of the study was to determine outcomes for the class as a whole.
IOLs
The IOLs used and their characteristics are listed in Table 1. The study size was limited by the number of mfIOLs donated: twenty mfIOLs from each manufacturer were available and used. An additional Synergy mfIOL was requested due to suboptimal patient selection and is discussed in the Results section below.
 |
Table 1 Characteristics of IOLs Used |
IOL Calculation
Biometry was performed with IOLMaster 700 (Carl Zeiss Meditec, Jena, Germany) and corneal tomography measured with Pentacam (Oculus Optikgeräte GmbH, Wetzlar, Germany). Multifocal IOLs were implanted where there was agreement between IOLMaster 700 and Pentacam for the axis of astigmatism (within 30°) and the total corneal power (within 0.5 D). Power calculations were made using the inbuilt Barrett TK Universal formula from the IOLMaster 700 for Mplus and Synergy IOLs, and using the online Zcalc calculator (https://zcalc.meditec.zeiss.com/) for the AT LISA IOL, as suggested by the IOL manufacturer. Target refraction was emmetropia in the MF30 and AT LISA IOL groups and the first plus target in the Synergy group, also as suggested by the IOL manufacturer. Toric IOLs were assigned to eyes with any astigmatism where calculations indicated benefit (including T2 toric IOLs) for the Synergy and AT LISA groups. In the MF30 group, toric IOLs were assigned to eyes with astigmatism of ≥0.75, as toric MF30 IOLs were custom made to the individual eye.
Surgical Technique
A single surgeon (CG) performed cataract extraction and IOL implantation after informed consent was obtained. Toric alignment was performed with the RoboMarker (Surgilūm, Wilmington, NC) or VERION Image Guided System (Alcon, San Diego, CA). A 2.4 mm temporal clear corneal incision was made and continuous curvilinear capsulorhexis performed. Phacoemulsification using a standard divide and conquer technique was performed and followed by coaxial irrigation and aspiration of the cortex. The IOL was inserted and aligned to the toric marking. After viscoelastic removal, the wounds were hydrated and intracameral cefazolin 2.5 mg in 0.1 mL was given. Patients were instructed to use topical chloramphenicol 0.5% eye drops four times daily for 14 days and dexamethasone 0.1% four times daily for 28 days. The second eye was completed within 1–3 months of the first operation.
Primary and Secondary Outcome Measures
Primary outcomes measured were uniocular and binocular-uncorrected distance (6 m; UDVA), intermediate (80 cm for AT LISA Tri or 66 cm for all others, as per the suggested distance from the IOL manufacturer; UIVA) and near (33 cm; UNVA) visual acuity. A backlit Snellen chart was used to measure distance acuity and handheld printed charts (Carl Zeiss Meditec, Jena, Germany) were used for intermediate and near acuity. Post-operative subjective refraction was performed, and the spherical equivalent (SE) and its difference from target SE was calculated.
Secondary outcomes included uniocular and binocular best corrected distance visual acuity (6m; CDVA), best corrected intermediate (80 cm or 66 cm as per IOL manufacturer; CIVA) and best corrected near (33 cm; CNVA) visual acuity. Root mean square higher-order aberrations (RMS HOA), and Chang-Wearing Chord (CWC; mm) were recorded. If the binocular CDVA was ≤ logMAR 0.2, defocus curves were measured in both eyes. The vision was best corrected for distance and defocus lenses from +1.00 D to – 4.00 D were applied, in steps of 0.50 D.
Quality of vision was measured at 1-month and 3-months using the self-administered Catquest-9SF (CQ)14 and Near Visual Acuity Questionnaire (NAVQ).15 Patients were also asked to rate the presence of dysphotopsias (halos, glare, starburst) and spectacle dependence (see Supplementary Tables 3 and 4).
Ophthalmic examination was performed at baseline and at 1 month post-operatively. Intraocular pressure, slit lamp, fundus examinations, biometry, and optical coherence tomography (OCT) were performed at both visits.
Data Collection and Statistical Method
Refractive, surgical, dysphotopsia and spectacle use data was entered into the Cataract & Lens Exchange Analysis & Register log (CLEARlog; HOYA Surgical Optics, UK).
Snellen units were converted to logMAR for statistical analysis. Descriptive statistics were calculated for all primary outcomes. The VA outcomes from the mfIOL groups were pooled, as the aim of the study was to examine the outcomes of the class as a whole and not to make comparisons between the mfIOL models.
Statistical analyses were performed on SPSS (SPSS v. 20.0, IBM Corp). The larger RMS HOA and CWC was selected for each patient and used to calculate the mean HOA RMS and mean CWC for each IOL group. Normality of data was assessed using the Shapiro–Wilk test. Normally distributed data was analysed using one-way analysis of variance, and data that was not normally distributed were analysed using the Kruskal–Wallis test. Statistical significance was set to a P value of 0.05.
Cost Analysis
A cost-analysis comparing the cost of bilateral cataract surgery with monofocal and mfIOLs, in addition to the projected cost of spectacles was conducted.16 Medical costs were determined using official Australian figures.17 IOL prostheses costs were taken from the current Australian Government Department of Health Prostheses List.18 The prosthesis cost for the mfIOLs ranged from $619 - $651 Australian Dollars (AUD) with a mean of $635 AUD, which was used as the mfIOL cost in this analysis. The cost of spectacles can vary considerably and there are no official recommendations for pricing, therefore the price for a pair of varifocal spectacles was sourced from a well-recognised Australian optometry chain and listed as $939 AUD.19 The spectacle price included an anti-reflective coating but no additional lens features were included. An analysis was conducted for glasses replacement every 1, 2 and 3 years to reflect the potential preferences of the patient20–23 for a total of 17 years, based on an average age at the time of cataract surgery of 68 years and a life expectancy of 85 years.24 Prices were adjusted for inflation where appropriate using the Reserve Bank of Australia Inflation Calculator.25 To facilitate comparison with other studies, the costs were converted to United States dollars (USD) using the exchange rate listed on 17th October 2023 (AUD1.00 = USD0.63).
Results
Optimised Surgical Pathway for mfIOLs
A total of 61 eyes from 31 patients were implanted with mfIOLs as part of the study. During the iterative process of protocol creation, mfIOLs were used in sentinel patients to determine if the protocol implemented to overcome the limitations of a public hospital to accommodate mfIOL use were effective. During this period, 2 patients had suboptimal mfIOL implantation: one patient was found to have vitelliform lesions after surgery, one had unexplained vision loss with the principal abnormality of ganglion cell layer thinning on OCT subsequently detected. These experiences led to refinement of the mfIOL protocol to include OCT as part of the pre-operative assessment, which is presented in Figure 1. With the protocol finalised, 58 eyes from 29 patients were implanted using the final pathway presented in Figure 1. Intra- and post-operative complications included 1 case of posterior capsule tear and 1 case of persistent cystoid macular oedema; both cases were excluded from the analysis. Fifty-four eyes from 27 patients were included in the analysis (Table 2). Over the study period, a total of 372 phacoemulsification procedures were performed at the hospital; mfIOL accounted for 7.3% of procedures performed.
 |
Table 2 Preoperative Characteristics and 1-Month Postoperative Monocular and Binocular Refractive Outcomes (Presented as Mean ± Standard Deviation Unless Otherwise Specified) |
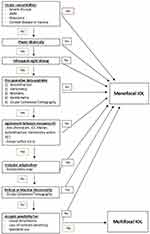 |
Figure 1 Optimised mfIOL selection pathway used by the trainee surgeon. |
Visual Acuity
Visual acuity outcomes are displayed in Table 2. All IOLs performed better for distance followed by intermediate and near foci, with binocular vision being superior to monocular vision. Overall, accuracy was excellent with 87% of eyes within 0.5D of target refraction and mean deviation from target refraction of only 0.2D.
Patient-Reported Outcomes
Patient reported dysphotopsias by group are displayed in Figure 2. Individual disturbances had a prevalence from 33% to 67%, with a mean prevalence of 56% for halos, 41% for glare and 52% for starburst.
 |
Figure 2 Any dysphotopsia, halos, glare or starburst in each IOL group (% participants per group). |
Complete spectacle independence (defined as a PROM rating of “never”) at distance, intermediate and near vision is displayed in Figure 3. Of note, no patients listed “mostly” or “always” for spectacle requirement for any visual distance, indicating the overall utility of the mfIOL. Satisfaction with overall vision (measured with CQ) and near vision specifically (measured with NAVQ) were rated highly in all IOL groups; all patients were either “completely” or “very” satisfied, see Figure 4. A complete summary of responses to PROMs is reported in supplementary tables 1–4.
 |
Figure 3 Self-rated spectacle dependence at distance, intermediate and near vision (% of participants per group). |
 |
Figure 4 Self-rated satisfaction with vision using the Catquest-9SF and Near Visual Acuity Questionnaire. |
Aberrations
In our sample, higher-order aberrations (RMS HOA) and the Chang-Warring Chord (CWC) were not associated with an increased risk of dysphotopsias (RMS HOA of 0.249 μm ± 0.085 μm vs 0.230 μm ± 0.064 μm for dysphotopsias vs no dysphotopsias; p = 0.664; CWC of 0.289 mm ± 0.156 mm vs 0.225 mm ± 0.128 mm, respectively; p = 0.612).
Cost-Analysis
Table 3 presents the economic costs of bilateral cataract surgery. The difference in price between multifocal and monofocal IOL conservatively equates to the price of a single pair of varifocal glasses. Assuming glasses are updated every year, mfIOL represents a saving to the patient of at least $15,185 AUD over 17 years, depending on the frames and lens options bought and excluding intangible costs such as the improvement in quality of life. However, these cost savings to the patient represent an increased cost to the health system that must pay for the advanced technology of a mfIOL. Our mfIOL cohort represented 7.3% of all cataract operations at Westmead during the study period; assuming between 5% and 10% of all public cataract patients were eligible for mfIOL implantation, this represents an increased cost of $1.1 to $2.3 million AUD per annum.
 |
Table 3 Economic Cost Analysis for Bilateral Cataract Surgery |
Discussion
In the correct patient, mfIOLs deliver improved intermediate and near visual acuity and greater spectacle independence when compared with monofocal IOLs.26 The challenge in the public sector, with variable measurement quality, significant time pressures that impact patient counselling, and multiple surgeons of various expertise influencing patient selection, is to implement a pathway that optimises patient outcomes while minimizing the potential risks and hazards inherent with such a mutable system. This pilot study proposes a pathway that overcomes many of these challenges. By empowering a senior trainee with adequate training and support, and utilising a stringent pathway that minimises the potential pitfalls inherent in mfIOL implantation,27 we were able to demonstrate favourable visual acuity outcomes with mean post-operative subjective refraction above benchmark standards observed in large-scale mfIOL or monofocal audits,28–30 high levels of spectacle independence and very high levels of patient satisfaction demonstrated through validated PROMs. This decision pathway can be adapted for use across a variety of settings and can aid ophthalmology departments in implementing mfIOL use in select patients.
There is value in considering mfIOL implantation in a public setting. The visual benefits afforded by mfIOLs are desirable as they improve the ease with which patients participate in near-vision related activities, social activities and overall activities, ultimately leading to improved quality of life.13 There are societal benefits of mfIOL use for patients who desire to return to work31 and a reduction in indirect costs of salary loss and unemployment.32 In older cohorts and aged care residents, cataract surgery has been shown to improve self-rated emotional wellbeing, mobility, independence33 and social interaction, in addition to visual function.34 Improvements in these domains can facilitate social connectedness with family, friends and community and potentially slow the progression of cognitive decline.35 Additionally, there is a reduced incidence of falls in the elderly36–38 which are associated with significant morbidity and mortality,39 and mfIOLs are associated with fewer falls than monofocal IOLs after first eye surgery.40 The findings highlight that the benefits of mfIOLs extend far beyond their visual outcomes and can have significant impacts on the quality of life and the everyday functioning of patients and their primary carers.
The challenge of funding innovative technologies and ensuring equitable access to health care within a resource-limited health system is universal,41–43 and has led to various price setting and funding models.44 This cost-analysis is one of the first to consider what the projected annual cost of mfIOLs would be to the Australian Health System. A prominent payment model that is used in European countries including Germany, Ireland and France45 utilises patient co-payments, where the health system funds the cost of the basic procedure and the patient funds the upgrade from a standard monofocal IOL to a mfIOL. This model is patient-centred, focused on patient outcomes and increases access and equity in health care delivery.46,47 A second model would see the additional prosthesis cost born by the health system and proposes allocating a fixed quota of mfIOLs for use per year. These outcomes could then be assessed to provide a larger evidence base to justify the investment, a practice that is emphasised in value-based healthcare models48,49 and is necessary for creating benchmarks for care.50 A third alternative would see mfIOLs tendered at a similar price to monofocal IOLs, with the cost absorbed by the IOL manufacturer, similar to strategies that have been described in orthopaedic surgery.51–53 There is benefit to the IOL manufacturer as surgeons become familiar with the brand and comfortable with the prosthesis, which can influence surgeon preferences for surgical devices54 and translate to increased usage outside the government funded health system. The three models presented are not mutually exclusive; elements of two or more can be combined to suit the individual hospital and health jurisdiction. The goal is to maintain cost neutrality while increasing access to newer technologies for patients and trainees and thus improving the standard of care.
Once the aforementioned barriers are addressed, it is necessary to allocate these valuable prostheses to patients who are most likely to benefit, which ensures their cost-effectiveness. This is where the selection pathway presented in Figure 1 is of value. Clinical selection pathways are an integral tool in helping standardise decision-making,55 reduce treatment error,56 ensure a high quality of care for all patients.57,58 They are effectively utilised in glaucoma and ocular hypertension management59 and can reduce the length of hospital admission for cataract patients.60 The surgical pathway proposed in Figure 1 is intentionally more stringent than is strictly necessary for mfIOL use to ensure each step in the pre-operative assessment is addressed to reduce error and optimise outcomes for every patient irrespective of the trainee’s prior experience, evidenced by the high visual acuity and PROMs achieved in our study. Further, our pathway can be used as a teaching guide for trainees to educate and expose them to the mfIOL process. Although experience in implanting presbyopia-correcting IOLs is not yet a necessary part of surgical training, they have become an increasing part of modern cataract surgery.8,9,61–63 It is likely that newly graduated surgeons will encounter these IOLs in their private practice, where expectations for optimal results are highest and there is limited or no senior supervision. Hands-on experience with presbyopia correcting IOLs can prepare trainees to identify patients who would benefit from them and to build technical skills and confidence in implanting them, with visual outcomes comparable to those of more experienced surgeons.64
This study had several limitations. The sample size was limited due to the availability of the mfIOL. Dysphotopsias were rated on a self-administered questionnaire, which is an inherently subjective. To improve the objectivity of this measure, dysphotopsias could be quantified using a visual simulator, which would also quantify their severity. We aimed to determine if mfIOLs could be effectively utilised in a public health system and with that in mind did not significantly alter the existing cataract assessment and follow-up protocols. This created limitations such as a short follow-up period where neuroadaptation for dysphotopsias was not assessed, and the use of an a-constant that was not optimised to the biometer65 or surgeon. Despite these limitations, our refractive outcomes were excellent implying that the pathway created can overcome the inherent limitations of a large tertiary referral public hospital.
To our knowledge, this is the first study to propose a selection pathway for mfIOLs that can be used by trainees in a public hospital setting. The development of the optimised mfIOL pathway was instrumental to the success of the trial demonstrating strong visual outcomes and PROMs. The additional cost for bilateral surgery is similar to the price of a single pair of glasses and their use should be considered in a government health system. The benefits to patients include improved visual acuity in the intermediate and near range, decreased spectacle dependence and high satisfaction, which is commonly linked to an improvement in quality of life.9,66 The benefit to surgical trainees is hands-on practice in the clinical assessment and management of mfIOL patients, including the nuances in selection, counselling and the management of patients with visual disturbances. Our pathway can be adapted to other training hospitals to suit the individual institutions equipment and needs and helped overcome the inherent limitations of a large, tertiary referral training hospital.
Abbreviations
mfIOL, Multifocal intraocular lens; IOL, Intraocular lens; UDVA, Uncorrected distance visual acuity;
UIVA, Uncorrected intermediate visual acuity; UNVA, Uncorrected near visual acuity; UDVA, Corrected distance visual acuity; UIVA, Corrected intermediate visual acuity; UNVA, Corrected near visual acuity; CQ, Catquest-9SF; NAVQ, Near visual acuity questionnaire; PROM, Patient reported outcome measure; RMS HOA, higher-order aberrations; CWC, Chang-Warring Chord; AUD, Australian Dollars; USD, American Dollars; CLEARlog, Cataract and lens exchange register.
Acknowledgments
This paper has been uploaded to ResearchGate as a preprint: https://www.researchgate.net/publication/384816131_The_Multifocal_Pathway_Trial_of_a_multifocal_intraocular_lens_selection_pathway_for_use_by_trainee_surgeons_in_an_Australian_Tertiary_Referral_Public_Hospital
Funding
LS is a recipient of University of Sydney RTP scholarship and stipend from HOYA optics. CS is a recipient of a National Health and Medical Research Council Investigator Grant (APP:1175949). The sponsor and funding organisations had no role in the design or conduct of this research.
Disclosure
Prof. Dr Andrew White reports grants from J&J, grants from Zeiss, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Kauh CY, Blachley TS, Lichter PR, Lee PP, Stein JD. Geographic Variation in the Rate and Timing of Cataract Surgery Among US Communities. JAMA Ophthalmol. 2016;134(3):267–276. doi:10.1001/jamaophthalmol.2015.5322
2. Congdon N, Vingerling JR, Klein BE, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol Apr. 2004;122(4):487–494.
3. AIHW. Admitted patient care 2018–19: Australian hospital statistics, Chapter 6: what procedures were performed? Availabe from: https://www.aihw.gov.au/getmedia/5afd4979-34e9-4753-9772-323be49fe96c/admitted-patient-care-2018-19-chapter-6-tables.xls.aspx.
4. Day AC, Donachie PHJ, Sparrow JM, Johnston RL. on behalf of all surgeons contributing towards The Royal College of Ophthalmologists’ National Ophthalmology D. The Royal College of Ophthalmologists’ National Ophthalmology Database study of cataract surgery: report 1, visual outcomes and complications. Eye. 2015;29(4):552–560. doi:10.1038/eye.2015.3
5. Lacy M, Kung T-PH, Owen JP, et al. Endophthalmitis Rate in Immediately Sequential versus Delayed Sequential Bilateral Cataract Surgery within the Intelligent Research in Sight (IRIS®) Registry Data. Ophthalmology. 2022;129(2):129–138. doi:10.1016/j.ophtha.2021.07.008
6. Low SAW, Braga-Mele R, Yan DB, El-Defrawy S. Intraoperative complication rates in cataract surgery performed by ophthalmology resident trainees compared to staff surgeons in a Canadian academic center. J Cataract Refract Surg. 2018;44(11):1344–1349. doi:10.1016/j.jcrs.2018.07.028
7. Zvorničanin J, Zvorničanin E. Premium intraocular lenses: the past, present and future. j Current Ophth. 2018;30(4):287–296. doi:10.1016/j.joco.2018.04.003
8. Rosen EMD, JLMDP A, HBMDP D, Dell SMD, Slade SMD. Efficacy and safety of multifocal intraocular lenses following cataract and refractive lens exchange: meta analysis of peer-reviewed publications. J Cataract Refract Surg. 2016;42(2):310–328. doi:10.1016/j.jcrs.2016.01.014
9. Khandelwal SS, Jun JJ, Mak S, Booth MS, Shekelle PG. Effectiveness of multifocal and monofocal intraocular lenses for cataract surgery and lens replacement: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2019;257(5):863–875. doi:10.1007/s00417-018-04218-6
10. Gil MA, Varón C, Cardona G, Buil JA. Visual acuity and defocus curves with six multifocal intraocular lenses. Int Ophthalmol. 2020;40(2):393–401. doi:10.1007/s10792-019-01196-4
11. Alió JL, Plaza-Puche AB, Piñero DP, Amparo F, Rodríguez-Prats JL, Ayala MJ. Quality of life evaluation after implantation of 2 multifocal intraocular lens models and a monofocal model. J Cataract Refract Surg Apr. 2011;37(4):638–648. doi:10.1016/j.jcrs.2010.10.056
12. Bamdad S, Ahmad Razavizadegan S, Farvardin M, Mohaghegh S. Vision-related Quality of Life after Bilateral Implantation of Monofocal and Multifocal Intraocular Lenses. J Ophthalmic Vis Res. 2022;17(1):19–26.
13. Javitt J, Brauweiler H-P, Jacobi KW, et al. Cataract extraction with multifocal intraocular lens implantation: clinical, functional, and quality-of-life outcomes: multicenter clinical trial in Germany and Austria. J Cataract Refract Surg. 2000;26(9):1356–1366. doi:10.1016/S0886-3350(00)00636-2
14. Gothwal VK, Wright TA, Lamoureux EL, Lundström M, Pesudovs K. Catquest questionnaire: re-validation in an Australian cataract population. Clin Exp Ophthalmol. 2009;37(8):785–794. doi:10.1111/j.1442-9071.2009.02133.x
15. Buckhurst PJ, Wolffsohn JS, Gupta N, Naroo SA, Davies LN, Shah S. Development of a questionnaire to assess the relative subjective benefits of presbyopia correction. J Cataract Refract Surg. 2012;38(1):74–79. doi:10.1016/j.jcrs.2011.07.032
16. Sanders GD, Neumann PJ, Basu A, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016;316(10):1093–1103. doi:10.1001/jama.2016.12195
17. Independent Hospital Pricing Authority (IHPA). National hospital cost data collection report: public sector, round 22 (financial year 2017–18). Canberra: IHPA; 2018 Available from: www.ihpa.gov.au/publications/national-hospital-cost-data-collection-datarequest-specifications-round-2.
18. Australian Government Department of Health. Prosthesis list part-A. Available from: https://www.health.gov.au/resources/publications/prescribed-list-of-medical-devices-and-human-tissue-products.
19. Ltd LRAP. SIMPLE PRICES. Availabe from: https://www.laubmanandpank.com.au/simple-prices.
20. Bala C, Athanasiov P, Holland J, Dhariwal M, Gupta A, Rathi H. A Cost-Effectiveness Analysis of AcrySof IQ Vivity Intraocular Lens (IOL) from Private Health Fund Perspective in Australia. Clin Ophthal. 16. (Auckland, NZ), 2022;2403–2412.
21. Berdahl J, Bala C, Dhariwal M, Rathi H, Gupta R, Jin G. Cost-benefit analysis of a trifocal intraocular lens versus a monofocal intraocular lens from the patient’s perspective in the United States. PLoS One. 2022;17(11):e0277093–e0277093. doi:10.1371/journal.pone.0277093
22. Cuq C, Lafuma A, Jeanbat V, Berdeaux G. A European Survey of Patient Satisfaction with Spectacles After Cataract Surgery and the Associated Costs in Four European Countries (France, Germany, Spain, and Italy). Ophthalmic Epidemiology. 2008;15(4):234–241. doi:10.1080/09286580801983237
23. Lafuma A, Laurendeau C, Lamerain E, Berdeaux G. Economics and attitudes regarding spectacles in daily life: a European perspective. Ophthalmic Epidemiol. 2009;16(4):218–223. doi:10.1080/09286580902999421
24. Statistics ABo. Life expectancy. Availabe from: https://www.abs.gov.au/statistics/people/population/life-expectancy/latest-release.
25. Australia RBo. Availabe from: https://www.rba.gov.au/calculator/financialYearDecimal.html.
26. de Silva SR, Evans JR, Kirthi V, Ziaei M, Leyland M, de Silva SR. Multifocal versus monofocal intraocular lenses after cataract extraction. Cochrane Database Syst Rev. 2016;2016(12):CD003169–CD003169. doi:10.1002/14651858.CD003169.pub4
27. Chen X. Successful Premium Multifocal IOL Surgery: key Issues and Pearls. Current Cataract Surg Techniq. 2021;7.
28. Gale RP, Saldana M, Johnston RL, Zuberbuhler B, McKibbin M. Benchmark standards for refractive outcomes after NHS cataract surgery. Eye. 2009;23(1):149–152. doi:10.1038/sj.eye.6702954
29. Kerr B, Diaper CJM, Rotchford AP. Cataract surgery refractive outcomes: representative standards in a National Health Service setting. British J Ophthalmology Apr. 2019.
30. Lundström M, Barry P, Henry Y, Rosen P, Stenevi U. Evidence-based guidelines for cataract surgery: guidelines based on data in the European Registry of Quality Outcomes for Cataract and Refractive Surgery database. J Cataract Refract Surg. 2012;38(6):1086–1093. doi:10.1016/j.jcrs.2012.03.006
31. Hu JQ, Sarkar R, Sella R, Murphy JD, Afshari NA. Cost-Effectiveness Analysis of Multifocal Intraocular Lenses Compared to Monofocal Intraocular Lenses in Cataract Surgery. Am J Ophthalmol. 2019;208:305–312. doi:10.1016/j.ajo.2019.03.019
32. Brown GC, Brown MM, Menezes A, Busbee BG, Lieske HB, Lieske PA. Cataract surgery cost utility revisited in 2012: a new economic paradigm. Ophthalmology. 2013;120(12):2367–2376. doi:10.1016/j.ophtha.2013.04.030
33. LAMOUREUX EL, HOOPER CY, L LIM, et al. Impact of Cataract Surgery on Quality of Life in Patients with Early Age-Related Macular Degeneration. Optometry Vision Sci. 2007;84(8):683–688. doi:10.1097/OPX.0b013e31812f755f
34. Owsley C, Gerald McGwin J, Scilley K, Meek GC, Seker D, Dyer A. Impact of cataract surgery on health-related quality of life in nursing home residents. Br J Ophthalmol. 2007;91(10):1359–1363. doi:10.1136/bjo.2007.118547
35. Paiva AF, Cunha C, Voss G, Delerue Matos A. The interrelationship between social connectedness and social engagement and its relation with cognition: a study using SHARE data. Ageing Soc. 2021;1–19.
36. Palagyi A, Morlet N, McCluskey P, et al. Visual and refractive associations with falls after first-eye cataract surgery. J Cataract Refract Surg. 2017;43(10):1313–1321. doi:10.1016/j.jcrs.2017.07.029
37. Harwood RH, Foss AJE, Osborn F, Gregson RM, Zaman A, Masud T. Falls and health status in elderly women following first eye cataract surgery: a randomised controlled trial. Br J Ophthalmol. 2005;89(1):53. doi:10.1136/bjo.2004.049478
38. Keay L, Ho KC, Rogers K, et al. The incidence of falls after first and second eye cataract surgery: a longitudinal cohort study. Med j Aust. 2022;217(2):94–99. doi:10.5694/mja2.51611
39. Health AIo, Welfare. Falls in older Australians 2019–20: hospitalisations and deaths among people aged 65 and over; 2022. Availabe from: https://www.aihw.gov.au/reports/injury/falls-in-older-australians-2019-20-hospitalisation.
40. Gadzhanova S, Gillam M, Roughead E. Risk of falls and injuries requiring hospitalisation after first-eye cataract surgery in elderly Australians. Acta Ophthalmologica. 2020;98(4):e495–e498. doi:10.1111/aos.14286
41. Institute of Medicine (US). Committee on Technological Innovation in Medicine; The Changing Economics of Medical Technology. In: The Diffusion of New Technology: Costs and Benefits to Health Care. Washington (DC): National Academies Press (US); 1991.
42. Cutler H. A roadmap towards scalable value-based payments in Australian Healthcare. Deeble Issues Brief 49. Australian Healthcare and Hospitals Association, Australia; 2022.
43. Harrill WC, Melon DE. A field guide to U.S. healthcare reform: the evolution to value-based healthcare. Laryngoscope Investig Otolaryngol. 2021;6(3):590–599. doi:10.1002/lio2.575
44. Barber SL, Lorenzoni L, Ong P. Institutions for health care price setting and regulation: a comparative review of eight settings. Int J Health Plann Manage. 2020;35(2):639–648. doi:10.1002/hpm.2954
45. Barber SL, Lorenzoni L, Ong P. Price setting and price regulation in health care: lessons for advancing Universal Health Coverage. World Health Organization. 2019.
46. Keely Anderson YDG, McCain K, Witkowski M. The Moment of Truth for Healthcare Spending: how Payment Models can Transform Healthcare Systems; 2023.
47. Barequet D, Tur-Sinai A, Barequet I. Health policy regulations pertaining to advanced surgical devices-their socio-economic effects on ophthalmology practice. Isr J Health Policy Res. 2019;8(1):13. doi:10.1186/s13584-019-0286-8
48. Robinson JC. Value-Based Purchasing For Medical Devices. Health Affa.
49. Nilsson K, Bååthe F, Andersson AE, Wikström E, Sandoff M. Experiences from implementing value-based healthcare at a Swedish University Hospital - an longitudinal interview study. BMC Health Serv Res. 2017;17(1):169. doi:10.1186/s12913-017-2104-8
50. Daniels K, van der Voort MBV R, Biesma DH, van der Nat PB. Five years’ experience with value-based quality improvement teams: the key factors to a successful implementation in hospital care. BMC Health Serv Res. 2022;22(1):1271. doi:10.1186/s12913-022-08563-5
51. Zuckerman JD, Kummer FJ, Frankel VH. The effectiveness of a hospital-based strategy to reduce the cost of total joint implants. J Bone Joint Surg Am. 1994;76(6):807–811. doi:10.2106/00004623-199406000-00003
52. Johnston DW, Beaupré LA, Davies DM, Hessels R. Reducing arthroplasty costs via vendor contracts. Can J Surg. 1999;42(6):445–449.
53. Boylan MR, Chadda A, Slover JD, Zuckerman JD, Iorio R, Bosco JA. Preferred Single-Vendor Program for Total Joint Arthroplasty Implants: surgeon Adoption, Outcomes, and Cost Savings. J Bone Joint Surg Am. 2019;101(15):1381–1387. doi:10.2106/JBJS.19.00008
54. Burns LR, Housman MG, Booth RE, Koenig AM. Physician preference items: what factors matter to surgeons? Does the vendor matter? Med Devices. 2018;11:39–49. doi:10.2147/MDER.S151647
55. Nippita TA, Porter M, Seeho SK, Morris JM, Roberts CL. Variation in clinical decision-making for induction of labour: a qualitative study. BMC Pregn Childbirth. 2017;17(1):317. doi:10.1186/s12884-017-1518-y
56. Rotter T, Kinsman L, James E, et al.Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. doi:10.1002/14651858.CD006632.pub2
57. Rotter T, de Jong RB, Lacko SE, et al. Clinical pathways as a quality strategy. Improving healthcare quality in Europe: characteristics, effectiveness and implementation of different strategies. Health Policy Ser. (53):12.
58. Lawal AK, Rotter T, Kinsman L, et al. What is a clinical pathway? Refinement of an operational definition to identify clinical pathway studies for a Cochrane systematic review. BMC Med. 2016;14(35):35. doi:10.1186/s12916-016-0580-z
59. Read S, Morgan J, Gillespie D, et al. Normalisation process theory and the implementation of a new glaucoma clinical pathway in hospital eye services: perspectives of doctors, nurses and optometrists. Report PLoS ONE. 2021; 16:e0255564.
60. Ling H, Zi-Feng Z, Yu-Sheng W, Hong-Fang C. Comprehensive analysis on quality of clinical pathway for hospitalized patients with cataract. Guo ji yan ke za zhi. 2014;14(10):1863–1865.
61. Cao K, Friedman DS, Jin S, et al. Multifocal versus monofocal intraocular lenses for age-related cataract patients: a system review and meta-analysis based on randomized controlled trials. Survey of Ophthalmology. 2019;64(5):647–658. doi:10.1016/j.survophthal.2019.02.012
62. Cho J-Y, Won YK, Park J, et al. Visual Outcomes and Optical Quality of Accommodative, Multifocal, Extended Depth-of-Focus, and Monofocal Intraocular Lenses in Presbyopia-Correcting Cataract Surgery: a Systematic Review and Bayesian Network Meta-analysis. JAMA Ophthalmology. 2022;140:1045. doi:10.1001/jamaophthalmol.2022.3667
63. Rho CR, Kim JH, Chung IK, et al. Cataract Surgery Practice in the Republic of Korea: a Survey of the Korean Society of Cataract and Refractive Surgery 2020. Korean J Ophthalmol. 2021;35(4):272–279. doi:10.3341/kjo.2020.0001
64. Roensch MAMD, Charton JWMD, Blomquist PHMD, Aggarwal NKMD, McCulley JPMD. Resident experience with toric and multifocal intraocular lenses in a public county hospital system. J Cataract Refract Surg. 2012;38(5):793–798. doi:10.1016/j.jcrs.2011.11.043
65. Aristodemou PF, Knox cartwright NEM, JMDF S, Johnston RLF. Intraocular lens formula constant optimization and partial coherence interferometry biometry: refractive outcomes in 8108 eyes after cataract surgery. J Cataract Refract Surg. 2011;37(1):50–62. doi:10.1016/j.jcrs.2010.07.037
66. Viljanen A, Koskela K, Koskela H, Tuuminen R, Uusitalo H. One-year Results of Health-related and Vision-related Quality of Life After Clear Lens Extraction and Multifocal Intraocular Lens Implantation. Am J Ophthalmol. 2021;227:240–244. doi:10.1016/j.ajo.2021.03.023
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

