Back to Journals » Journal of Inflammation Research » Volume 17
The NRF-2/HO-1 Signaling Pathway: A Promising Therapeutic Target for Metabolic Dysfunction-Associated Steatotic Liver Disease
Authors Li N , Hao L, Li S, Deng J, Yu F , Zhang J , Nie A, Hu X
Received 6 August 2024
Accepted for publication 18 October 2024
Published 3 November 2024 Volume 2024:17 Pages 8061—8083
DOI https://doi.org/10.2147/JIR.S490418
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Adam Bachstetter
Na Li,1,2 Liyuan Hao,1,2 Shenghao Li,1,2 Jiali Deng,1,2 Fei Yu,1,2 Junli Zhang,3 Aiyu Nie,1,2 Xiaoyu Hu2
1Department of Clinical Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China; 2Department of Infectious Diseases, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China; 3Jiangsu Provincial Hospital of Traditional Chinese Medicine, Nanjing, People’s Republic of China
Correspondence: Xiaoyu Hu, Hospital of Chengdu University of Traditional Chinese Medicine, No. 39 Shi-Er-Qiao Road, Chengdu, Sichuan Province, 610075, People’s Republic of China, Email [email protected]
Abstract: Metabolic dysfunction-associated steatotic liver disease (MASLD) is a progressive liver disorder with a rising prevalence. It begins with lipid accumulation in hepatocytes and gradually progresses to Metabolic-associated steatohepatitis (MASH), fibrosis, cirrhosis, and potentially hepatocellular carcinoma (HCC). The pathophysiology of MASLD is complex and involves multiple factors, with oxidative stress playing a crucial role. Oxidative stress drives the progression of MASLD by causing cellular damage, inflammatory responses, and fibrosis, making it a key pathogenic mechanism. The Nuclear Factor Erythroid 2-Related Factor 2 / Heme Oxygenase-1 (Nrf2/HO-1) signaling axis provides robust multi-organ protection against a spectrum of endogenous and exogenous insults, particularly oxidative stress. It plays a pivotal role in mediating antioxidant, anti-inflammatory, and anti-apoptotic responses. Many studies indicate that activating the Nrf2/HO-1 signaling pathway can significantly mitigate the progression of MASLD. This article examines the role of the Nrf2/HO-1 signaling pathway in MASLD and highlights natural compounds that protect against MASLD by targeting Nrf2/HO-1 activation. The findings indicate that the Nrf2/HO-1 signaling pathway holds great promise as a therapeutic target for MASLD.
Keywords: the NRF-2/HO-1 signaling pathway, antioxidants, anti-inflammatory, natural compounds, non-alcoholic fatty liver disease
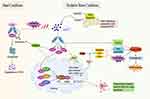
Graphical Abstract:

Introduction
Non-alcoholic fatty liver disease (NAFLD) is a condition characterized by excessive fat accumulation in the liver, excluding alcohol and other specific causes of liver damage.1 To more accurately reflect the metabolic basis of the disease, an international expert panel introduced a new term in 2020: Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD), which replaced the old term “Non-Alcoholic Fatty Liver Disease” (NAFLD).2 In 2023, a multi-society Delphi consensus statement on the new nomenclature for fatty liver disease introduced the term Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) and formally discontinued the use of NAFLD.3 Based on disease progression, MASLD can be classified into four distinct stages: simple steatosis, Metabolic-Associated Steatohepatitis (MASH), fibrosis, and cirrhosis.4 The clinical manifestations of MASLD are diverse, with the majority of patients being asymptomatic. However, some patients may report symptoms such as fatigue, discomfort in the right upper quadrant, hepatomegaly, acanthosis nigricans, and lipomas.5 In more severe cases, symptoms like jaundice, loss of appetite, nausea, and vomiting may occur.6 Furthermore, MASLD is closely associated with metabolic and systemic diseases such as cardiovascular disease, obesity, type 2 diabetes, and sarcopenia. These conditions are not only common complications of MASLD,7–9 but may also exacerbate its progression, leading to further deterioration of the patient’s condition.10–12
MASLD has become the most common liver disease worldwide and is expected to be the leading cause of end-stage liver disease in the coming decades.13,14 According to statistics, the global prevalence of MASLD ranges from 25% to 34%, with 30% in Europe, 35% in South and North America, and 29.29% in Asia. Notably, the prevalence in Southeast Asia is as high as 42%.15–18 The mortality rate for individuals with MASLD is 1.6 times higher than that of the general population,19–21 with an all-cause mortality rate of 12.60 per 1000 person-years, posing a significant public health crisis.22 Among the management strategies for MASLD, weight loss is considered one of the most effective measures; however, patients often struggle to maintain long-term adherence in practice.23,24 Pharmacological and surgical treatments have also been shown to improve patients’ metabolic conditions and increase survival rates effectively. Commonly used medications include insulin sensitizers such as pioglitazone,23 statins,24 and vitamin E,25 which can effectively alleviate fatty liver and inflammatory responses, particularly in patients with metabolic syndrome. Resmetirom, as an emerging drug, selectively activates the hepatic thyroid hormone receptor β, which can improve metabolic function, reduce hepatic fat accumulation, alleviate liver inflammation, enhance liver fibrosis, and potentially increase insulin sensitivity.26 Weight loss surgery, such as gastric bypass, significantly reduces weight, improves liver function, and decreases the incidence of MASH and liver fibrosis.27 Moreover, liver transplantation is the ultimate treatment option for end-stage MASH.28,29 Although these treatments have demonstrated certain clinical efficacy, the overall management of MASLD patients remains suboptimal due to the lack of early effective diagnostic tools and targeted therapeutic agents.30 Therefore, there is an urgent need to intensify efforts to develop new treatment strategies for MASLD.
The “second hit” theory is a classic pathogenic mechanism of MASLD, where the “first hit” typically refers to insulin resistance leading to excessive fat accumulation in the liver (hepatic steatosis), oxidative stress is considered the “second hit”, significantly promoting the development of steatohepatitis.31 In recent years, the “multiple-hit” hypothesis has increasingly replaced the “second hit” theory, gaining widespread acceptance.32 This hypothesis posits that MASLD is influenced by multiple pathological factors that collectively lead to hepatic fat deposition, inflammatory response, and fibrosis.33,34
Nevertheless, oxidative stress remains one of the key factors in alleviating MASLD.35–37 When the balance between oxidants and antioxidants is disrupted, oxidative stress can trigger lipotoxicity, lipid peroxidation, endoplasmic reticulum (ER) stress, and mitochondrial dysfunction, while also increasing the production of inflammatory cytokines. This cascade of events leads to inflammatory and fibrotic responses, ultimately progressing to MASH and end-stage liver disease.37,38
The nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) pathway is a critical signaling mechanism in the body’s response to oxidative stress.39 Nrf2, a basic leucine zipper transcription factor, is essential for regulating the cellular antioxidant response and is widely expressed in various tissues and cells.40,41 Research indicates that Nrf2 exerts dual protective effects in MASLD: (1) it negatively regulates genes that promote hepatic lipid accumulation. (2) it eliminates Reactive Oxygen Species (ROS) and electrophiles produced by lipid peroxidation, thereby preventing oxidative stress and mitochondrial dysfunction in hepatocytes.42 The downstream effector of Nrf2, HO-1, plays a crucial role in anti-inflammatory, antioxidant, and anti-apoptotic processes.43 HO-1 significantly improves MASLD by enhancing mitochondrial function, inhibiting ferroptosis, reducing ROS production, and suppressing inflammatory responses.44–47 Qiu et al48 demonstrated that activating the Nrf2/HO-1 pathway can regulate antioxidant enzyme levels, eliminate lipid peroxides, maintain the balance between oxidation and antioxidation, reduce weight gain, and improve lipid metabolic dysfunction, effectively suppressing the progression of MASLD both in vitro and in vivo. Additionally, Qiao et al49 indicated that iNOS upregulates HO-1 expression by promoting Nrf2 nuclear translocation, thereby protecting the liver from MASH damage. Once HO-1 expression is inhibited, the protective effects of iNOS on hepatocytes diminish. This underscores the potential of the Nrf2/HO-1 signaling pathway as a therapeutic target for the progression of MASLD.
Based on the aforementioned information, the Nrf2/HO-1 pathway presents significant potential as a therapeutic target for non-alcoholic fatty liver disease (NAFLD). Recent studies indicate that various natural compounds, such as Aucubin,50 Gastrodin,51 and Ganoderma lucidum polysaccharides,52 can activate the Nrf2/HO-1 pathway, exerting antioxidant, anti-inflammatory, and lipid metabolism-improving effects that effectively inhibit the pathological progression of NAFLD. Therefore, we propose targeting the Nrf2/HO-1 pathway as an innovative approach to intervene in NAFLD, which may not only slow disease progression but also improve associated complications. In the future, as our understanding of this pathway deepens, Nrf2/HO-1 targeted therapies are expected to serve as a valuable complement to existing treatments, opening new avenues for NAFLD research.
Heading
Oxidative Stress and MASLD
In 1998, British scholar Day first proposed the “two-hit theory” of MASLD pathogenesis, which has been widely accepted within the scientific community. According to this hypothesis, the “first hit” is peripheral insulin resistance leading to the accumulation of free fatty acids in the liver, resulting in hepatic steatosis. This condition predisposes the liver to further damage, preparing it for the subsequent “second hit”. The “second hit” involves oxidative stress and the production of pro-inflammatory cytokines, which contribute to the progression of steatohepatitis.31 However, as research has advanced, scientists have increasingly recognized that the complexity of this disease far exceeds the “two-hit” theory. The “multiple-hit theory” has been proposed and gradually replaced the “two-hit” hypothesis as a widely accepted perspective.32 This theory posits that the onset and progression of MASLD are not triggered by two singular events but rather involve a synergistic interplay of various pathological factors, including but not limited to genetic susceptibility, epigenetic regulation, metabolic dysregulation, gut microbiota imbalance, insulin resistance, lipid peroxidation, and immune responses,33,34 these factors interact in complex ways, promoting the accumulation of fat in the liver, inflammatory responses, and the acceleration of fibrosis. The “two-hit” hypothesis emphasizes that steatosis occurs first, followed by hepatitis and fibrosis triggered by oxidative stress and other factors, while the “multiple-hit” hypothesis posits that multiple pathogenic factors can act simultaneously or sequentially to lead to the onset and progression of MASLD.
Despite the complex pathogenesis of NAFLD involving multiple factors, oxidative stress is consistently recognized as a crucial contributor to disease progression.53 Oxidative stress arises from an imbalance between the production and elimination of ROS,54 which serves as a primary cause of both hepatic and extrahepatic injury.55 Clinical studies have shown that compared to the normal group, patients with NAFLD exhibit significantly higher body mass index, cholesterol levels, and transaminases, while levels of glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), glutathione reductase (GR), and glutathione peroxidase (GPX) are significantly lower.56,57 The decreased activity of these antioxidant enzymes leads to exacerbated oxidative stress, further aggravating liver cell damage, inflammation, and fibrosis.37,58,59 Additionally, oxidative stress initiates a series of pathophysiological changes in MASLD, including mitochondrial dysfunction, ER stress, disturbances in iron metabolism, disruption of the gut-liver axis, insulin resistance, and endothelial dysfunction.55
Nrf2/HO-1 Signaling Pathway
Nrf2 is pivotal in cellular antioxidant defense, ensuring redox homeostasis.60 This key transcription factor dissociates from its inhibitory protein, Kelch-like ECH-related protein 1 (Keap1), during oxidative stress and relocates to the nucleus. In the nucleus, Nrf2 engages with the Antioxidant Response Element (ARE) to activate the transcription of multiple antioxidant genes, including HO-1. HO-1 plays a crucial role in shielding cells from damage caused by ROS and is essential for anti-inflammatory, antioxidant, and anti-apoptotic functions.61,62 As the primary regulatory mechanism for cellular protection against oxidative stress, the Nrf2/HO-1 pathway is essential in managing MASLD.63
The Structure and Properties of Nrf2
Nrf2 also referred to as Nfe2l2, a transcription factor, is encoded by the NFE2L2 gene on chromosome 2q31.2.64 This protein is part of the cap “n” collar (CNC) basic leucine zipper (bZIP) family of transcription factors.65 Nrf2 is ubiquitously expressed in multiple tissues, including the liver, kidneys, spleen, and heart. It is critically involved in protecting these tissues from oxidative stress and chemical-induced cellular damage.66 Nrf2 consists of 605 amino acids and features seven distinct structural domains, which are sequentially arranged from the N-terminus to the C-terminus as Neh2, Neh4, Neh5, Neh7, Neh6, Neh1, and Neh3.67 Each domain fulfills a specific and irreplaceable function.68 The Neh2 domain, located at the N-terminus of Nrf2, contains two highly conserved amino acid sequences: 29DLG (low affinity) and 79ETGE (high affinity)69, which are the two sites that bind to the Kelch domain of the Nrf2 repressor protein (Keap1).70,71 Nrf2 is subject to ubiquitination by the Keap1-Cullin3 E3 ubiquitin ligase, which results in its degradation via the proteasome system.72 The Neh4 and Neh5 domains, together with the C-terminal Neh3, act as transactivation domains for Nrf2, binding to coactivators.73,74 The Neh7 domain, serving a negative regulatory function, binds to retinoid X receptor alpha (RXR α) and inhibits the transcriptional activity of Nrf2.75 The Neh6 domain is a key negative regulatory domain that mediates the ubiquitination and proteasomal degradation of Nrf2, containing the amino acid sequences DSGIS338 and DSAPGS378,76 which are recognized by GSK-3/β-TrCP and targeted for degradation by the Cullin1/Rbx1 complex.77 Lastly, the Neh1 domain, defined by its conserved CNC and bZIP structures, is crucial for Nrf2 to bind to small Maf proteins in the nucleus, facilitating the formation of dimers that recognize and attach to the DNA sequences of target genes,78 particularly the Antioxidant Response Element (ARE)79(Figure 1).
The Structure and Characteristics of HO-1
HO-1 is a 32 kDa stress-inducible protein that belongs to the heme oxygenase family.80 It is a type II detoxifying enzyme regulated by Nrf2 and serves as the rate-limiting enzyme in the oxidative degradation of heme into free iron, carbon monoxide (CO), and biliverdin.81 HO-1 is highly expressed in various digestive organs, including the gastrointestinal tract, pancreas, and liver.82 HO-1 is an enzyme encoded by the HMOX-1 gene, located on human chromosome 22q12.3.83 The gene spans approximately 13,148 bp and contains 5 exons and 4 introns, accompanied by 3 regulatory regions. A proximal regulatory region is positioned at about −0.3 Kb, while two distal enhancer regions, designated E1 and E2, are located at approximately −4 Kb and −10 Kb, respectively.84–86 These regulatory regions contain numerous transcription factor binding sites, such as hypoxia-inducible factor 1 (HIF-1), nuclear factor kappa B (NF-κB), activator protein 1 (AP-1), stress response element (StRE), metal response element (MtRE), and heat shock element (HSE).87,88 These regulatory elements facilitate the transcriptional response of the HMOX-1 gene to various oxidative and inflammatory stimuli.87 StRE is the primary sequence motif, functioning analogously to the Maf response element (MARE) and the antioxidant response element (ARE).89 Among the transcription factors, nuclear erythroid 2-related factor (Nrf2) and BTB and CNC homolog 1 (Bach1) (transcriptional repressors of HMOX1) play key roles in HMOX1 regulation, regulating gene expression by activating and repressing its transcription, respectively.90 HO-1 is present at low levels in most mammalian tissues but plays a crucial protective role in cells, including anti-inflammatory, antioxidant, anti-apoptotic, and pro-angiogenic.91 Upon activation, HO-1 degrades heme into biliverdin, carbon monoxide (CO), and ferrous iron. Biliverdin is subsequently converted to bilirubin by biliverdin reductase, which scavenges or neutralizes ROS, thus mitigating oxidative stress.82,92 As a gaseous signaling molecule, CO exerts various effects in signal transduction, including vasodilation, anti-inflammatory responses, anti-apoptotic effects, and the promotion of angiogenesis.93 Additionally, the activation of HO-1 upregulates the expression of ferritin, which binds ferrous iron, thereby reducing oxidative stress94(Figure 2).
The Nrf2/HO-1 Signaling Pathway
The Nrf2/HO-1 signaling axis plays a pivotal role in maintaining homeostasis by regulating calcium ion influx, oxidative stress, ferroptosis, pyroptosis, autophagy, and programmed cell necrosis.95 Nrf2, as an endogenous antioxidant transcription regulator, serves as a primary modulator of cellular defense and survival.96,97 Under physiological conditions, Nrf2 binds to Keap1 to form a complex sequestered in the cytoplasm.68,98 Keap1 interacts with Cullin3 and the Rbx1 subunit of the E3 ubiquitin ligase complex to form a protein complex. This complex binds to the Neh2 domain of Nrf2, leading to its ubiquitination and maintaining Nrf2 in a low activity state within the cell. Keap1 acts as a redox sensor, and upon oxidative thiol modification, it loses its ability to inhibit Nrf2. HO-1 is a crucial mediator of the antioxidant and anti-inflammatory effects of Nrf2.99 Under normal conditions, the chromatin structure of HO-1 remains in a pre-activated state, with its transcription inhibited by Bach1.100 Specifically, the HMOX1 promoter is suppressed by the Bach1/Maf dimer binding to the StRE element.101
Upon exposure to oxidative stress or other pathological stimuli, the regulatory cysteine thiols on Keap1 react with ROS, leading to the dissociation of Keap1 from Nrf2. This dissociation allows Nrf2 to translocate into the nucleus. Inside the nucleus, Nrf2 forms a heterodimer with small Maf proteins (sMaf) and Jun bZip transcription factors, collectively referred to as the Nrf2-Maf complex, this complex accurately recognizes sequences containing antioxidant response elements (ARE) and binds to the Neh4 and Neh5 domains of Nrf2.102–104 Through interactions with cAMP response element-binding protein (CREB) and other transcriptional activators, the Nrf2-mediated transcription process is initiated, the regulation of downstream gene expression includes HO-1, NAD(P)H quinone oxidoreductase 1 (NQO1), glutamate cysteine ligase (GCL), peroxiredoxin (Prdx), SOD, CAT, GR, and glutathione peroxidase (GSH-Px).63 These genes facilitate the clearance of ROS and other harmful substances, promoting mechanisms of antioxidant response, anti-inflammation, and anti-apoptosis.63 Studies have shown that, in addition to Keap1, several protein kinase signaling pathways can induce Nrf2 phosphorylation and participate in its transcriptional regulation.60 Among these, mitogen-activated protein kinases (MAPK), protein kinase C (PKC), and phosphoinositide 3-kinase (PI3K) positively regulate NRF2 activity, while glycogen synthase kinase 3 (GSK-3) negatively regulates NRF2 activity through phosphorylation at different sites.105,106 Additionally, oxidative stress can cause heme to be released from hemoproteins. Free heme binds to Bach1, inducing conformational changes in its structure. This results in the dissociation of Bach1 from StRE, thereby increasing the transcription of HO-1 within cell107(Figure 3).
The Role of the Nrf2/HO-1 Signaling Pathway in MASLD
MASLD is a prevalent chronic liver disorder primarily driven by oxidative stress and lipid peroxidation, which result in cellular damage, apoptosis, inflammation, and fibrosis. Improving lipid metabolism and reducing hepatic oxidative stress and inflammatory responses are considered effective strategies for preventing and treating MASLD.108 Nrf2 is an intracellular transcription regulator, with HO-1 as one of its most significant downstream products. The cascade reaction between Nrf2 and HO-1 is crucial for the body’s anti-inflammatory and antioxidant systems.39 Studies have demonstrated that the Nrf2/HO-1 pathway is involved in regulating every stage of the MASLD spectrum.47,109 Moreover, a range of natural compounds has demonstrated potential therapeutic effects across various stages of MASLD, including simple steatosis, MASH, fibrosis, cirrhosis, and even HCC. This is largely attributed to their ability to activate the Nrf2/HO-1 signaling pathway (Table 1).
 |
Table 1 Natural Compounds Modulating the Nrf2/HO-1 Pathway to Mitigate MASLD |
Impact of the Nrf2/HO-1 Signaling Pathway on Simple Steatosis
Nrf2/HO-1 Signaling Pathway and Simple Steatosis
The early stages of MASLD are characterized by the accumulation of lipids within hepatocytes. This condition results from increased fatty acid uptake and synthesis, coupled with decreased fatty acid oxidation and removal.138 Reducing oxidative stress and inflammation is crucial for the effective prevention and treatment of high-fat diet (HFD)-induced steatosis.139 The Nrf2/HO-1 pathway is a crucial mechanism for mitigating oxidative stress. Activation of this pathway effectively reduces ROS production in hepatocytes and inhibits RIP3 expression, thereby decreasing inflammation and lipid deposition.140 As a negative regulator of genes associated with hepatic steatosis, Nrf2 suppresses key lipid-synthesizing enzymes, reduces hepatic fat storage, and participates in fatty acid metabolism.42 Its activation enhances lipid breakdown and inhibits de novo lipogenesis,141 thus reducing lipid accumulation and oxidative stress in HFD-fed mice.142 Increased HO-1 activity also aids in the treatment of MASLD by significantly impacting hepatic steatosis and preventing its progression to MASH, cirrhosis, and related complications.45,47
Natural Compounds Modulating the Nrf2/HO-1 Pathway to Mitigate Simple Steatosis
Linalool inhibits lipid accumulation and oxidative stress by activating the Nrf2/HO-1 signaling pathway, thus preventing HFD-induced MASLD.110 Aucubin (AU) significantly reduces lipid accumulation and oxidative stress by activating the Nrf2/HO-1 and AMPK signaling pathways.50 Gastrodin (GSTD) promotes the phosphorylation of Nrf2 at serine 40, stimulating Nrf2 nuclear translocation and increasing hepatic expression of HO-1. Concurrently, it activates AMPK, thereby inhibiting oxidative stress and inflammatory responses, and improving hepatic steatosis.51 Ganoderma lucidum polysaccharides (GDLP) activate Nrf2, inducing the expression of antioxidant enzymes such as HO-1, SOD, CAT, and GSH-Px, reducing MDA levels, and inhibiting hepatic steatosis, oxidative stress, and inflammation in db/db mice.52 Limonene upregulates the hepatic Nrf2/HO-1 signaling pathway, reduces ROS accumulation, inhibits macrophage infiltration, and decreases the expression of pro-inflammatory cytokines, thereby exerting anti-lipid deposition, antioxidant, and anti-inflammatory effects.112 Hesperetin can alleviate hepatic steatosis, oxidative stress, inflammatory cell infiltration, and fibrosis by activating the PI3k/AKT-Nrf2 pathway, up-regulating antioxidant levels (SOD/GPX/GR/GCLC/HO-1), decreasing ROS production, inhibiting NF-κB activation, and decreasing the secretion of inflammatory factors (TNF-α and IL-6).123 Wogonoside activates the Nrf2/HO-1 pathway to inhibit oxidative stress and reduces inflammation by inhibiting the NF-κB pathway to protect MASLD mice from liver injury, and significantly reduces liver mass, liver index, and levels of LDL, TG, and TC in the wogonoside group as compared to the MASLD group.129 Scutellarin can alleviate non-alcoholic fatty liver disease (NAFLD) by activating the Nrf2/HO-1 signaling pathway, thereby reducing oxidative stress.127,128 Naringin activates the Nrf2/HO-1 pathway, inhibits the NF-κB/TNF-α pathway, and reduces endogenous triglyceride synthesis, thereby preventing the progression of MASLD.108 Geniposide (GEN) activates Nrf2 expression, increasing cytoplasmic HO-1 protein levels and significantly reducing lipid accumulation in HepG2 cells. Knockdown of Nrf2 diminishes the liver’s antioxidant capacity and nullifies GEN’s beneficial effects on TC, TG, and LDL levels.120 Dehydroabietic acid (DA) binds to Keap1, activates Nrf2-ARE, and promotes the expression of HO-1, GSH, and GPX4, thereby inhibiting ROS accumulation and reducing MDA levels, which alleviates HFD-induced MASLD. Additionally, DA inhibits ferroptosis by upregulating key genes, including ferroptosis suppressor protein 1 (FSP1).113
Impact of the Nrf2/HO-1 Signaling Pathway on MASH
Nrf2/HO-1 Signaling Pathway and MASH
Non-alcoholic steatohepatitis (NASH), also known as metabolic dysfunction-associated steatohepatitis (MASH), is a severe inflammatory form of MASLD.143 MASH is characterized by fatty infiltration, oxidative stress, and necrotic inflammation of the liver, with or without fibrosis144,145 significantly increasing the risk of progressing to severe liver disease.146 Nrf2 regulates the recruitment of inflammatory cells and induces antioxidant responses to counteract the inflammatory process.147 Studies have shown that Nrf2 activation can inhibit NF-κB activity, thereby reducing the expression of pro-inflammatory cytokines and inflammatory mediators such as IL-1, IL-6, IL-10, TNF-α, COX, NO, and iNOS, as well as adhesion molecules like ICAM-1 and VCAM-1.148,149 During this process, the Nrf2/HO-1 pathway plays a crucial role by reducing ROS levels through the inhibition of hepatic oxidative stress. Since ROS and their oxidized lipid peroxides can activate NF-κB, leading to enhanced pro-inflammatory signaling, the Nrf2/HO-1 pathway mitigates this inflammation by decreasing the production of ROS and lipid peroxides.150,151 Clearly, the activation of the Nrf2/HO-1 pathway not only enhances the activity of antioxidant enzymes (such as superoxide dismutase and CAT), reducing oxidative damage to hepatocytes but also effectively suppresses the excessive expression of NF-κB-dependent inflammatory factors, thereby alleviating the inflammatory response.123 Okada et al152 discovered that mice with Nrf2 gene deficiency displayed increased oxidative stress, steatosis, inflammation, fibrosis, and ferroptosis under a methionine- and choline-deficient (MCD) diet, resulting in the rapid progression of MASH.153 In contrast, activating Nrf2 can inhibit MASH by reducing oxidative stress and ameliorating lipotoxicity, inflammation, ER stress, and iron overload.154 Furthermore, upregulation of the HO-1 gene can alleviate hepatic steatosis and necroinflammatory responses, significantly lowering serum ALT and AST levels in MASH mice.155 Li et al156 further confirmed that upregulating the Nrf2/HO-1 signaling pathway effectively inhibits oxidative stress and inflammatory damage and significantly reduces lipid levels, as well as levels of ALT, AST, MDA, IL-1β, and TNF-α. Additionally, it enhances the activity of SOD and GSH-Px, thereby alleviating hepatic steatosis, ballooning degeneration, and inflammation. These findings indicate that activation of the Nrf2/HO-1 pathway is crucial for combating MASH.49
Natural Compounds Modulating the Nrf2/HO-1 Pathway to Mitigate MASH
Aloin exerts protective effects against MASH by mediating antioxidant, anti-inflammatory, and anti-apoptotic actions through activation of the Nrf2/HO-1 pathway.130 Capillin can inhibit PA-mediated oxidative stress in hepatocytes by promoting the expression of Nrf2/HO-1 and reduce PA-mediated hepatocyte apoptosis by suppressing the NLRP3-ASC-Caspase1 inflammasome. Through these mechanisms, Capillin effectively ameliorates hepatic fat accumulation, oxidative stress, and liver injury in MASH mice.131 Flavones of hawthorn leafonon mitigate oxidative stress-induced cellular damage and liver inflammation by promoting Nrf2/HO-1 expression and inhibiting COX-2 overexpression, thus preventing the development of MASH.124 Xin et al125 found that baicalin alleviates oxidative stress by activating the Nrf2/HO-1 pathway in the liver and inhibits NF-κB activation, reducing the expression of IL-6 and TNF-α, thus suppressing inflammation. Additionally, baicalin regulates liver mitochondrial function. Further research by Shi et al126 revealed that baicalin reduces NLRP3/Caspase1/GSDMD levels by activating Nrf2/HO-1 expression, thereby inhibiting pyroptosis and decreasing lipid accumulation and inflammation in the liver tissues of MASH mice.
Impact of the Nrf2/HO-1 Signaling Pathway on Hepatic Fibrosis
Nrf2/HO-1 Signaling Pathway and Hepatic Fibrosis
Hepatic fibrosis is a reversible wound-healing response and degenerative disease caused by the excessive deposition of extracellular matrix proteins such as collagen. This response represents the liver’s mechanism to counteract prolonged injury or disease, aiming to contain the damaged area and promote healing. However, persistent fibrosis can progress to cirrhosis and liver failure, ultimately necessitating a liver transplant.157 Oxidative stress is a major factor in hepatocyte injury and may exacerbate inflammation and fibrosis in MASH patients.158 Activation of Nrf2 and HO-1 can effectively ameliorate hepatic fibrosis.159–161 Khadrawy et al162 demonstrated that activating the Nrf2/HO-1 signaling pathway improves oxidative stress, inflammation, and hepatic fibrosis in rats. This improvement is evidenced by decreased levels of serum transaminases, ALP, γGT, and bilirubin, inhibition of MDA, NF-κB p65, and inflammatory cytokine expression, and reduced histological changes and collagen accumulation in the liver, thereby ameliorating hepatic fibrosis.
Natural Compounds Modulating the Nrf2/HO-1 Pathway to Mitigate Hepatic Fibrosis
Schisandrin B activates nuclear Nrf2 and Nrf2-related antioxidant genes (HO-1, NQO1, GCLC), thereby inhibiting oxidative stress-mediated hepatocyte injury in fibrotic rats. Additionally, it suppresses hepatic stellate cell (HSC) activation by inhibiting the TGF-β/Smad signaling pathway.132 Raspberry extract (RBE) upregulates the Nrf2/HO-1 and PPAR-γ signaling pathways, eliminates oxidative stress, induces HSC apoptosis, and alleviates hepatic fibrosis.134 Tanshinol not only enhances SOD and GSH-Px levels and reduces MDA levels via the Nrf2/HO-1 signaling pathway, thereby inhibiting oxidative stress-induced damage, but also inhibits the NF-κB signaling pathway, reducing the expression of inflammatory factors such as TGF-β and TNF-α. Consequently, tanshinol lowers the levels of alanine transaminase, aspartate transaminase, total bilirubin, hyaluronic acid, type IV collagen, laminin (LN), and procollagen III peptide (PIIIP) in fibrotic rats, significantly inhibiting hepatic fibrosis.136 Salvianolic acid A increases SOD and GSH-Px levels, decreases MDA levels, inhibits inflammation and oxidative stress, and ameliorates CCl4-induced hepatic fibrosis by modulating the Nrf2/HO-1, NF-κB/IκBα, p38 MAPK, and JAK1/STAT3 signaling pathways.137 Ginsenoside Rg1 promotes the nuclear translocation of Nrf2 and enhances the expression of antioxidant enzymes such as HO-1, SOD, and GSH-Px, thereby inhibiting hepatic fibrosis.135 Betulin (BE) upregulates Nrf2 and HO-1 expression in a dose-dependent manner and inhibits NF-κB gene expression, effectively reducing serum lipid and transaminase levels in HFD-fed rats, thereby preventing hepatic fat accumulation, steatosis, and fibrosis.114 Fan et al115 demonstrated that Asiatic acid (AA) effectively ameliorates CCl4-induced hepatic fibrosis in rats. The underlying mechanisms include the activation of the Nrf2/ARE signaling pathway, resulting in increased nuclear Nrf2 expression and decreased cytoplasmic Nrf2 levels. This upregulates Nrf2 target proteins such as HO-1 and NQO-1, effectively countering oxidative stress-induced liver damage. Additionally, AA inhibits the NF-κB/IκBα and JAK1/STAT3 signaling pathways, reducing the release of inflammatory factors and the activation of HSCs, further preventing the progression of hepatic.
Impact of the Nrf2/HO-1 Signaling Pathway on Liver Cirrhosis and Liver Cancer
Nrf2/HO-1 Signaling Pathway in Liver Cirrhosis and Liver Cancer
Liver cirrhosis and liver cancer represent the advanced stages of MASLD. Cirrhosis significantly impairs liver structure and function, potentially resulting in liver failure, portal hypertension, and liver cancer. Oxidative stress is a key mechanism driving hepatic lipid metabolism disorders, cirrhosis, and fibrosis.163 Studies have indicated that Nrf2-deficient mice are more prone to developing MASH accompanied by cirrhosis.164 During liver cirrhosis, the activation of the ER stress response pathway, specifically XBP1-Hrd, upregulates Hrd1 transcription and inhibits Nrf2-mediated antioxidant responses, thereby promoting the progression of cirrhosis.165 However, enhanced Nrf2 can prevent cirrhosis by reducing ROS levels and subsequently decreasing HSCs activation.166 Additionally, once induced, HO-1 can exert protective effects in cirrhosis.167 Xue et al demonstrated that HO-1 protects hepatocytes in cirrhotic rats from liver I/R injury by reducing oxidative stress, apoptosis, and inflammation.168
Hepatocellular carcinoma (HCC) is the most prevalent form of primary liver cancer, constituting over 80% of all cases.169 Research indicates that Astragaloside IV facilitates the phosphorylation of Nrf2, enhances the activation of HO-1 expression, mitigates oxidative stress, and inhibits the progression of HCC.170 Furthermore, Purslane (Portulaca oleracea) has been shown to effectively suppress the phosphorylation of PI3K, Akt, mTOR, NF-κB, and IκBα, upregulate the expression of Nrf2 and HO-1, exhibit anti-inflammatory and antioxidant properties, reduce the levels of ALT, AST, IL-6, IL-1β, TNF-α, and MDA in HCC mice, restore SOD activity, and markedly ameliorate liver pathological alterations.171 Additionally, HO-1 inhibits the proliferation and migration of HCC in vivo by downregulating the levels of miR-30d and miR-107 through its metabolites, thereby significantly suppressing HCC progression.172 Hence, the activation of the Nrf2/HO-1 signaling pathway presents a potential therapeutic approach for HCC.
However, the application of the Nrf2/HO-1 signaling pathway in MASLD-related cirrhosis and liver cancer remains controversial. Some researchers suggest that Nrf2/HO-1 activation may, in certain cases, increase portal vein pressure and cause abnormal visceral hemodynamics in cirrhotic rats with portal hypertension.173 Moreover, sustained activation of Nrf2 may exacerbate the progression of HCC,174 and promote chemoresistance in cancer cells.174 Additionally, short (GT)n variants in the HO-1 gene may increase susceptibility to cirrhosis and cancer.175 Therefore, the molecular mechanisms and potential advantages and disadvantages of the Nrf2/HO-1 antioxidant pathway in cirrhosis and liver cancer require further investigation and discussion.
Natural Compounds Modulating the Nrf2/HO-1 Pathway to Liver Cirrhosis and Liver Cancer
Astragaloside IV (AS-IV), one of the primary active components of Astragalus, possesses pharmacological properties including anti-inflammatory and anticancer effects. Zhang et al117 demonstrated that AS-IV activates the pSmad3C/3L and Nrf2/HO-1 pathways, thereby inhibiting collagen fiber deposition and the development of primary liver cancer. Furthermore, the Nrf2/HO-1 pathway is notably more effective in contributing to the anti-HCC effects of AS-IV compared to pSmad3C/3L.118
Conclusion
MASLD is a complex lipotoxic disease characterized by hepatic steatosis and oxidative stress, which plays a central role in its pathophysiology. The Nrf2/HO-1 pathway is a critical antioxidant mechanism in MASLD. Activation of Nrf2 regulates the gene expression of various endogenous antioxidant enzymes, including HO-1, NQO1, SOD, potentially alleviating hepatic steatosis, MASH,153 liver fibrosis,176 and the onset and progression of HCC.177 HO-1, regulated by Nrf2, is essential for the removal of toxic heme. Upregulation of HO-1 can reverse the progression of hepatic steatosis, liver fibrosis, cirrhosis, and systemic complications.47 Furthermore, targeting the Nrf2/HO-1 pathway can not only effectively inhibit the progression of MASLD but also serve as an effective treatment for related complications such as obesity, type 2 diabetes, and sarcopenia.178,179
Studies have shown that various natural compounds mitigate the progression of MASLD by activating the Nrf2/HO-1 pathway, which regulates lipid metabolism, oxidative stress, inflammatory responses, and apoptosis in hepatocytes (Table 1). Additionally, other drugs also modulate MASLD through the Nrf2/HO-1 pathway. For example, liraglutide180 and lansoprazole181 have been shown to improve hepatic lipid metabolism and oxidative stress via the activation of the Nrf2/HO-1 pathway. Moreover, traditional Chinese medicine formulations, such as Hedansanqi Tiaozhi Tang (HTT)48 and Di’ao Xinxuekang (DXXK),156 alleviate MASLD by activating Nrf2/HO-1, thus inhibiting oxidative stress and inflammation.
In summary, the Nrf2/HO-1 pathway regulates various pathological processes, including lipid metabolism, oxidative stress, inflammatory response, and apoptosis, effectively inhibiting the pathological progression of MASLD. Targeting this pathway holds promise for providing a more comprehensive treatment strategy for MASLD compared to existing drugs. However, the development of this pathway as a therapeutic target still faces numerous challenges. Firstly, precisely activating Nrf2 is a critical issue, as excessive activation may lead to adverse effects such as abnormal cell proliferation and immune suppression, which need to be addressed in future studies. Secondly, while various natural compounds have shown potential therapeutic effects on MASLD in animal models, their clinical translation still faces significant challenges. Currently, there is a lack of large-scale, randomized controlled clinical trials to validate the efficacy and safety of these compounds. Additionally, optimizing their dosage and administration routes to ensure effectiveness and long-term safety across different individuals remains an urgent issue to be resolved.
Future research should focus on the precise regulatory mechanisms of the Nrf2/HO-1 pathway, exploring how to selectively activate Nrf2 without eliciting adverse effects. Additionally, the clinical applicability of natural compounds in MASLD needs to be validated through further large-scale clinical trials. By conducting in-depth studies on the Nrf2/HO-1 pathway, optimizing natural compounds, and investigating their synergistic effects with existing medications, we can develop more personalized and safe treatment strategies for MASLD. This endeavor will require the collaborative efforts of more researchers in the field.
Consent for Publication
All authors have agreed to the publication of this manuscript.
Funding
The present study was financially supported by the National Natural Science Foundation of China (No. 81973840), Sichuan Provincial Administration of Traditional Chinese Medicine Major Science and Technology projects (2021XYCZ004).
Disclosure
The authors declare that no commercial or financial relationships exist that could be interpreted as potential conflicts of interest in relation to this research.
References
1. H GS, A WS, Elsherbiny DA, et al. Non-alcoholic fatty liver disease: an overview of risk factors, pathophysiological mechanisms, diagnostic procedures, and therapeutic interventions [J]. Life Sci. 2021;271:119220. doi:10.1016/j.lfs.2021.119220
2. Eslam M, N NP, K SS, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement [J]. J Hepatol. 2020;73(1):202–209. doi:10.1016/j.jhep.2020.03.039
3. E RM, V LJ, Ratziu V, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78(6):1966–1986. doi:10.1097/HEP.0000000000000520
4. Ratziu V, Bellentani S, Cortez-Pinto H, et al. A position statement on NAFLD/NASH based on the EASL 2009 special conference [J]. J Hepatol. 2010;53(2):372–384.
5. R BB, J FM, G JC, et al. Nonalcoholic steatohepatitis: an expanded clinical entity [J]. Gastroenterology. 1994;107(4):1103–1109. doi:10.1016/0016-5085(94)90235-6
6. Yuan S, M ZH, X LJ, et al. Gasotransmitters in non-alcoholic fatty liver disease: just the tip of the iceberg [J]. Eur J Pharmacol. 2023;954:175834. doi:10.1016/j.ejphar.2023.175834
7. Gutiérrez-Cuevas J, Santos A, Armendariz-Borunda J. Pathophysiological molecular mechanisms of obesity: a link between MAFLD and NASH with cardiovascular diseases [J]. Int J Mol Sci. 2021;22(21):11629. doi:10.3390/ijms222111629
8. Godoy-Matos AF, Júnior W S S, Valerio CM. NAFLD as a continuum: from obesity to metabolic syndrome and diabetes [J]. Diabetol Metab Syndr. 2020;12:60. doi:10.1186/s13098-020-00570-y
9. Tarantino G, Sinatti G, Citro V, et al. Sarcopenia, a condition shared by various diseases: can we alleviate or delay the progression? Intern Emerg Med. 2023;18(7):1887–1895. doi:10.1007/s11739-023-03339-z
10. Mathews SE, Kumar RB, Shukla AP. Nonalcoholic steatohepatitis, obesity, and cardiac dysfunction [J]. Curr Opini Endoc Diab Obesi. 2018;25(5):315–320. doi:10.1097/MED.0000000000000432
11. Kim H, S LD, H AT, et al. Metabolic spectrum of liver failure in type 2 diabetes and obesity: from NAFLD to NASH to HCC [J]. Int J Mol Sci. 2021;22(9).
12. Iwaki M, Kobayashi T, Nogami A, et al. Impact of sarcopenia on non-alcoholic fatty liver disease [J]. Nutrients. 2023;15(4). doi:10.3390/nu15040891
13. Miao L, Targher G, D BC, et al. Current status and future trends of the global burden of MASLD [J]. Trend Endocrinol metabol. 2024;35(8):697–707. doi:10.1016/j.tem.2024.02.007
14. Younossi Z, M AQ, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention [J]. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi:10.1038/nrgastro.2017.109
15. Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention [J]. Nat Rev Gastroenterol Hepatol. 2021;18(4):223–238. doi:10.1038/s41575-020-00381-6
16. Alexander M, K LA, Fairburn-Beech J, et al. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease [J]. BMC Med. 2018;16(1):130. doi:10.1186/s12916-018-1103-x
17. L TM, H NC, Q HD, et al. Global incidence and prevalence of nonalcoholic fatty liver disease [J]. Clin Molecular Hepatol. 2023;29(Suppl):S32–s42. doi:10.3350/cmh.2022.0365
18. H LM, Yeo YH, Li X, et al. Global NAFLD prevalence: a systematic review and meta-analysis [J]. Clin Gastroent Hepatol. 2022;20(12):2809–17.e28. doi:10.1016/j.cgh.2021.12.002
19. Musso G, Gambino R, Cassader M, et al. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity [J]. Ann Med. 2011;43(8):617–649. doi:10.3109/07853890.2010.518623
20. Bril F, J SJ, M BA, et al. Hepatic steatosis and insulin resistance, but not steatohepatitis, promote atherogenic dyslipidemia in NAFLD [J]. J Clin Endocrinol Metab. 2016;101(2):644–652. doi:10.1210/jc.2015-3111
21. Jepsen P, Vilstrup H, Mellemkjaer L, et al. Prognosis of patients with a diagnosis of fatty liver--a registry-based cohort study [J]. Hepato-Gastroenterol. 2003;50(54):2101–2104.
22. M YZ, Golabi P, M PJ, et al. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77(4):1335–1347. doi:10.1097/HEP.0000000000000004
23. Della Pepa G, Russo M, Vitale M, et al. Pioglitazone even at low dosage improves NAFLD in type 2 diabetes: clinical and pathophysiological insights from a subgroup of the TOSCA.IT randomised trial [J]. Diabetes Res Clin Pract. 2021;178:108984. doi:10.1016/j.diabres.2021.108984
24. Maroni L, Guasti L, Castiglioni L, et al. Lipid targets during statin treatment in dyslipidemic patients affected by nonalcoholic fatty liver disease [J]. Am J Med Sci. 2011;342(5):383–387. doi:10.1097/MAJ.0b013e318213e526
25. El Hadi H, Vettor R, Rossato M. Vitamin E as a treatment for nonalcoholic fatty liver disease: reality or myth? Antioxidants. 2018;7(1). doi:10.3390/antiox7010012
26. Karim G, Bansal MB. Resmetirom: an orally administered, smallmolecule, liver-directed, β-selective thr agonist for the treatment of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis [J]. TouchREVIE Endocrin. 2023;19(1):60–70. doi:10.17925/EE.2023.19.1.60
27. Głuszyńska P, Lemancewicz D, B DJ, et al. Non-Alcoholic Fatty Liver Disease (NAFLD) and bariatric/metabolic surgery as its treatment option: a review [J]. J Clin Med. 2021;10(24). doi:10.3390/jcm10245721
28. Sasaki A, Nitta H, Otsuka K, et al. Bariatric surgery and non-alcoholic Fatty liver disease: current and potential future treatments [J]. Front Endocrinol. 2014;5:164. doi:10.3389/fendo.2014.00164
29. Saeed N, Glass L, Sharma P, et al. Incidence and risks for nonalcoholic fatty liver disease and steatohepatitis post-liver transplant: systematic review and meta-analysis [J]. Transplantation. 2019;103(11):e345–e54. doi:10.1097/TP.0000000000002916
30. Zhou J, Zhou F, Wang W, et al. Epidemiological Features of NAFLD From 1999 to 2018 in China. Hepatology. 2020;71(5):1851–1864. doi:10.1002/hep.31150
31. Day CP, James OF. Steatohepatitis: a tale of two ”hits”? Gastroenterology. 1998;114(4):842–845. doi:10.1016/S0016-5085(98)70599-2
32. Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) [J]. Metabolism. 2016;65(8):1038–1048. doi:10.1016/j.metabol.2015.12.012
33. Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease [J]. Cell. 2021;184(10):2537–2564. doi:10.1016/j.cell.2021.04.015
34. Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52(5):1836–1846. doi:10.1002/hep.24001
35. Hong T, Chen Y, Li X, et al. The role and mechanism of oxidative stress and nuclear receptors in the development of NAFLD [J]. Oxid Med Cell Longev. 2021;2021:6889533. doi:10.1155/2021/6889533
36. Martín-Fernández M, Arroyo V, Carnicero C, et al. Role of oxidative stress and lipid peroxidation in the pathophysiology of NAFLD [J]. Antioxidants. 2022;11(11). doi:10.3390/antiox11112217
37. C A-OJ, Wu Z, Geng Y, et al. Role of oxidative stress in the pathogenesis of non-alcoholic fatty liver disease: implications for prevention and therapy [J]. Antioxidants. 2021;10(2). doi:10.3390/antiox10020174
38. Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis [J]. Free Radic Biol Med. 2012;52(1):59–69. doi:10.1016/j.freeradbiomed.2011.10.003
39. Loboda A, Damulewicz M, Pyza E, et al. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism [J]. CMLS. 2016;73(17):3221–3247. doi:10.1007/s00018-016-2223-0
40. Xu L, Nagata N, Ota T. Impact of glucoraphanin-mediated activation of Nrf2 on non-alcoholic fatty liver disease with a focus on mitochondrial dysfunction [J]. Int J Mol Sci. 2019;20(23):5920. doi:10.3390/ijms20235920
41. Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress [J]. Free Radic Biol Med. 2009;47(9):1304–1309. doi:10.1016/j.freeradbiomed.2009.07.035
42. Chambel SS, Santos-Gonçalves A, Duarte TL. The dual role of Nrf2 in nonalcoholic fatty liver disease: regulation of antioxidant defenses and hepatic lipid metabolism [J]. Biomed Res. Int. 2015;2015:597134. doi:10.1155/2015/597134
43. Biswas C, Shah N, Muthu M, et al. Nuclear heme oxygenase-1 (HO-1) modulates subcellular distribution and activation of Nrf2, impacting metabolic and anti-oxidant defenses [J]. J Biol Chem. 2014;289(39):26882–26894. doi:10.1074/jbc.M114.567685
44. Hinds T D SDE Jr, Tiribelli C. Powering the powerhouse: heme oxygenase-1 regulates mitochondrial function in non-alcoholic fatty liver disease (NAFLD). Acta Physiologica. 2023;237(3):e13931. doi:10.1111/apha.13931
45. E SD, D HT Jr. Natural product heme oxygenase inducers as treatment for nonalcoholic fatty liver disease [J]. Int J Mol Sci. 2020;21(24):9493.
46. Yuan X, Li L, Zhang Y, et al. Heme oxygenase 1 alleviates nonalcoholic steatohepatitis by suppressing hepatic ferroptosis [J]. Lipids Health Dis. 2023;22(1):99. doi:10.1186/s12944-023-01855-7
47. Sasson A, Kristoferson E, Batista R, et al. The pivotal role of heme Oxygenase-1 in reversing the pathophysiology and systemic complications of NAFLD [J]. Arch Biochem Biophys. 2021;697:108679. doi:10.1016/j.abb.2020.108679
48. Qiu M, Xiao F, Wang T, et al. Protective effect of Hedansanqi Tiaozhi Tang against non-alcoholic fatty liver disease in vitro and in vivo through activating Nrf2/HO-1 antioxidant signaling pathway [J]. Phytomedicine. 2020;67:153140. doi:10.1016/j.phymed.2019.153140
49. Qiao Y, Li X, Zhang X, et al. Hepatocellular iNOS protects liver from NASH through Nrf2-dependent activation of HO-1 [J]. Biochem. Biophys. Res. Commun. 2019;514(2):372–378. doi:10.1016/j.bbrc.2019.04.144
50. Shen B, Zhao C, Wang Y, et al. Aucubin inhibited lipid accumulation and oxidative stress via Nrf2/HO-1 and AMPK signalling pathways [J]. J Cell & Mol Med. 2019;23(6):4063–4075. doi:10.1111/jcmm.14293
51. L QL, Yu B, Li Z, et al. Gastrodin ameliorates oxidative stress and proinflammatory response in nonalcoholic fatty liver disease through the AMPK/Nrf2 pathway [J]. Phytoth Resea. 2016;30(3):402–411. doi:10.1002/ptr.5541
52. N LH, L ZL, Y ZD, et al. Ganoderma lucidum polysaccharides ameliorates hepatic steatosis and oxidative stress in db/db mice via targeting nuclear factor E2 (Erythroid-Derived 2)-Related Factor-2/Heme Oxygenase-1 (HO-1) pathway [J]. Med Sci. 2020;26:e921905. doi:10.12659/MSM.921905
53. Paradies G, Paradies V, M RF, et al. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease [J]. World J Gastroenterol. 2014;20(39):14205–14218. doi:10.3748/wjg.v20.i39.14205
54. Bovi A P D, Marciano F, Mandato C, et al. Oxidative stress in non-alcoholic fatty liver disease. Mini Rev Front Medic. 2021;8:595371.
55. Masarone M, Rosato V, Dallio M, et al. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease [J]. Oxid Med Cell Longev. 2018;2018:9547613. doi:10.1155/2018/9547613
56. Madan K, Bhardwaj P, Thareja S, et al. Oxidant stress and antioxidant status among patients with nonalcoholic fatty liver disease (NAFLD) [J]. J Clin Gastroenterol. 2006;40(10):930–935. doi:10.1097/01.mcg.0000212608.59090.08
57. S DK, Balakrishnan V, Mukherjee S, et al. Evaluation of blood oxidative stress-related parameters in alcoholic liver disease and non-alcoholic fatty liver disease [J]. Scand J Clin Lab Invest. 2008;68(4):323–334. doi:10.1080/00365510701673383
58. Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance [J]. Nature. 2006;440(7086):944–948. doi:10.1038/nature04634
59. Zhao Q, Liu J, Deng H, et al. Targeting mitochondria-located circRNA SCAR Alleviates NASH via reducing mROS output [J]. Cell. 2020;183(1):76–93.e22. doi:10.1016/j.cell.2020.08.009
60. Liu S, Pi J, Zhang Q. Signal amplification in the KEAP1-NRF2-ARE antioxidant response pathway [J]. Redox Biol. 2022;54:102389. doi:10.1016/j.redox.2022.102389
61. Campbell NK, Fitzgerald HK, Dunne A. Regulation of inflammation by the antioxidant haem oxygenase 1 [J]. Nat Rev Immunol. 2021;21(7):411–425. doi:10.1038/s41577-020-00491-x
62. Wang H, Cheng Q, Bao L, et al. Cytoprotective role of heme oxygenase-1 in cancer chemoresistance: focus on antioxidant, antiapoptotic, and pro-autophagy properties. Antioxidants. 2023;12(6):1217. doi:10.3390/antiox12061217
63. Zhang Q, Liu J, Duan H, et al. Activation of Nrf2/HO-1 signaling: an important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress [J]. J Adv Res. 2021;34:43–63. doi:10.1016/j.jare.2021.06.023
64. Moi P, Chan K, Asunis I, et al. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region [J]. Proceedings Nat Acad Sci United States of America. 1994;91:(21). 9926–9930. doi:10.1073/pnas.91.21.9926
65. Basu P, L AD, Maier C, et al. The Effects of Nuclear Factor Erythroid 2 (NFE2)-Related Factor 2 (Nrf2) activation in preclinical models of peripheral neuropathic pain. Antioxidants. 2022;11(2):430. doi:10.3390/antiox11020430
66. Pan Y, Zeng X, Wen S, et al. Characterization of the cap ‘n’ collar isoform c gene in Spodoptera frugiperda and its association with superoxide dismutase [J]. Insects. 2020;11(4):221. doi:10.3390/insects11040221
67. Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism [J]. Trends Biochem Sci. 2014;39(4):199–218. doi:10.1016/j.tibs.2014.02.002
68. Tonelli C, Chio IIC, Tuveson DA. Transcriptional regulation by Nrf2 [J]. Antioxid Redox Sign. 2018;29(17):1727–1745. doi:10.1089/ars.2017.7342
69. Jung KA, Kwak MK. The Nrf2 system as a potential target for the development of indirect antioxidants [J]. Molecules. 2010;15(10):7266–7291. doi:10.3390/molecules15107266
70. Itoh K, Wakabayashi N, Katoh Y, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain [J]. Genes Dev. 1999;13(1):76–86.
71. I TK, Katoh Y, Kusunoki H, et al. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model [J]. Mol Cell Biol. 2006;26(8):2887–2900. doi:10.1128/MCB.26.8.2887-2900.2006
72. A AD, Goldstein M, Albanyan H, et al. Discovery of direct inhibitors of Keap1-Nrf2 protein-protein interaction as potential therapeutic and preventive agents [J]. Acta pharmaceutica Sinica B. 2015;5(4):285–299. doi:10.1016/j.apsb.2015.05.008
73. Zhang J, Hosoya T, Maruyama A, et al. Nrf2 Neh5 domain is differentially utilized in the transactivation of cytoprotective genes [J]. Biochem J. 2007;404(3):7266–7291. doi:10.1042/BJ20061611
74. Nioi P, Nguyen T, J SP, et al. The carboxy-terminal Neh3 domain of Nrf2 is required for transcriptional activation [J]. Mol Cell Biol. 2005;25(24):11629–11906. doi:10.1128/MCB.25.24.10895-10906.2005
75. Wang H, Liu K, Geng M, et al. RXRα inhibits the NRF2-ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer Res. 2013;73(10):3097–3108. doi:10.1158/0008-5472.CAN-12-3386
76. Chowdhry S, Zhang Y, McMahon M, et al. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity [J]. Oncogene. 2013;32(32):3765–3781. doi:10.1038/onc.2012.388
77. Cuadrado A. Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/β-TrCP [J]. Free Radic Biol Med. 2015;88(Pt B):147–157. doi:10.1016/j.freeradbiomed.2015.04.029
78. Motohashi H, Katsuoka F, D EJ, et al. Small Maf proteins serve as transcriptional cofactors for keratinocyte differentiation in the Keap1-Nrf2 regulatory pathway [J]. Proceedings Nat Acad Sci United States of America, 2004, 101(17): 6379–6384.
79. Itoh K, Chiba T, Takahashi S, et al. An Nrf2/small Maf heterodimer mediates the induction of Phase II detoxifying enzyme genes through antioxidant response elements [J]. Biochem. Biophys. Res. Commun. 1997;236(2):313–322. doi:10.1006/bbrc.1997.6943
80. Keyse SM, Tyrrell RM Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite [J]. Proceedings Nat Acad Sci United States of America, 1989, 86(1): 99–103.
81. Qiao H, Sai X, Gai L, et al. Association between heme oxygenase 1 gene promoter polymorphisms and susceptibility to coronary artery disease: a HuGE review and meta-analysis [J]. Amer j epidem. 2014;179(9):1039–1048. doi:10.1093/aje/kwu024
82. D P-PJ, Moreno-SanJuan S, Á C, et al. Heme oxygenase-1 in gastrointestinal tract health and disease [J]. Antioxidants. 2020;9(12). doi:10.3390/antiox9121214
83. K KR, N NC, Kutty G, et al. Increased expression of heme oxygenase-1 in human retinal pigment epithelial cells by transforming growth factor-beta [J]. J Cell Physiol. 1994;159(2):371–378. doi:10.1002/jcp.1041590221
84. Bian C, Zhong M, F NM, et al. A novel heme oxygenase-1 splice variant, 14kDa HO-1, promotes cell proliferation and increases relative telomere length [J]. Biochem. Biophys. Res. Commun. 2018;500(2):429–434. doi:10.1016/j.bbrc.2018.04.096
85. Alam J, Camhi S, Choi AM. Identification of a second region upstream of the mouse heme oxygenase-1 gene that functions as a basal level and inducer-dependent transcription enhancer [J]. J Biol Chem. 1995;270(20):11977–11984. doi:10.1074/jbc.270.20.11977
86. Alam J, Igarashi K, Immenschuh S, et al. Regulation of heme oxygenase-1 gene transcription: recent advances and highlights from the International Conference (Uppsala, 2003) on Heme Oxygenase [J]. Antioxid Redox Sign. 2004;6(5):924–933. doi:10.1089/ars.2004.6.924
87. Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications [J]. Physiol Rev. 2006;86(2):583–650. doi:10.1152/physrev.00011.2005
88. Lavrovsky Y, L SM, D LR, et al. Identification of binding sites for transcription factors NF-kappa B and AP-2 in the promoter region of the human heme oxygenase 1 gene [J]. Proceedings Nat Acad Sci United States of America, 1994, 91(13): 5987–5991.
89. Inamdar NM, Ahn YI, Alam J. The heme-responsive element of the mouse heme oxygenase-1 gene is an extended AP-1 binding site that resembles the recognition sequences for MAF and NF-E2 transcription factors [J]. Biochem. Biophys. Res. Commun. 1996;221(3):570–576. doi:10.1006/bbrc.1996.0637
90. Alam J, Cook JL. How many transcription factors does it take to turn on the heme oxygenase-1 gene? [J]. Am J Respir Cell Mol Biol. 2007;36(2):166–174. doi:10.1165/rcmb.2006-0340TR
91. Zhou X, Yuan W, Xiong X, et al. HO-1 in bone biology: potential therapeutic strategies for osteoporosis [J]. Front Cell Develop Biol. 2021;9:791585. doi:10.3389/fcell.2021.791585
92. Nitti M, Ivaldo C, Traverso N, et al. Clinical significance of heme oxygenase 1 in tumor progression [J]. Antioxidants. 2021;10(5):789. doi:10.3390/antiox10050789
93. Motterlini R, Nikam A, Manin S, et al. HYCO-3, a dual CO-releaser/Nrf2 activator, reduces tissue inflammation in mice challenged with lipopolysaccharide [J]. Redox Biol. 2019;20:334–348. doi:10.1016/j.redox.2018.10.020
94. D FC, R JS, Sawa A, et al. Haem oxygenase-1 prevents cell death by regulating cellular iron [J]. Nat Cell Biol. 1999;1(3):152–157. doi:10.1038/11072
95. Zhang X, Yu Y, Lei H, et al. The Nrf-2/HO-1 signaling axis: a ray of hope in cardiovascular diseases [J]. Cardiol Res Pract. 2020;2020:5695723. doi:10.1155/2020/5695723
96. Jaramillo MC, Zhang DD. The emerging role of the Nrf2-Keap1 signaling pathway in cancer [J]. Genes Dev. 2013;27(20):2179–2191. doi:10.1101/gad.225680.113
97. He F, Ru X, Wen T. NRF2, a transcription factor for stress response and beyond [J]. Int J Mol Sci. 2020;21(13):4777. doi:10.3390/ijms21134777
98. Suzuki T, Yamamoto M. Molecular basis of the Keap1-Nrf2 system [J]. Free Radic Biol Med. 2015;88(Pt B):93–100. doi:10.1016/j.freeradbiomed.2015.06.006
99. Ryter SW. Heme oxgenase-1, a cardinal modulator of regulated cell death and inflammation [J]. Cells. 2021;10(3):515. doi:10.3390/cells10030515
100. Sun J, Brand M, Zenke Y, et al. Heme regulates the dynamic exchange of Bach1 and NF-E2-related factors in the Maf transcription factor network [J]. Proceedings Nat Acad Sci United States of America, 2004, 101(6): 1461–1466.
101. Reichard JF, Motz GT, Puga A. Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1 [J]. Nucleic Acids Res. 2007;35(21):7074–7086. doi:10.1093/nar/gkm638
102. Katsuoka F, Yamamoto M. Small Maf proteins (MafF, MafG, MafK): history, structure and function [J]. Gene. 2016;586(2):197–205. doi:10.1016/j.gene.2016.03.058
103. Zhang J, Zhang T, Zeng S, et al. The role of Nrf2/smaf signalling in retina ageing and retinal diseases [J]. Biomedicines. 2023;11(6):1512. doi:10.3390/biomedicines11061512
104. Hirotsu Y, Katsuoka F, Funayama R, et al. Nrf2-MafG heterodimers contribute globally to antioxidant and metabolic networks [J]. Nucleic Acids Res. 2012;40(20):10228–10239. doi:10.1093/nar/gks827
105. Liu T, F LY, L ZJ, et al. Regulation of Nrf2 by phosphorylation: consequences for biological function and therapeutic implications [J]. Free Radic Biol Med. 2021;168:129–141. doi:10.1016/j.freeradbiomed.2021.03.034
106. Kang KW, Lee SJ, Kim SG. Molecular mechanism of nrf2 activation by oxidative stress [J]. Antioxid Redox Sign. 2005;7(11–12):1664–1673. doi:10.1089/ars.2005.7.1664
107. Sadeghi M, Fathi M, Gholizadeh navashenaq J, et al. The prognostic and therapeutic potential of HO-1. Leukemia MDS Cell Commu Signalin. 2023;21(1):57. doi:10.1186/s12964-023-01074-8
108. Pengnet S, Sumarithum P, Phongnu N, et al. Naringin attenuates fructose-induced NAFLD progression in rats through reducing endogenous triglyceride synthesis and activating the Nrf2/HO-1 pathway [J]. Front Pharmacol. 2022;13:1049818. doi:10.3389/fphar.2022.1049818
109. N BV, Moola A, Serviddio G, et al. Nuclear factor erythroid 2-related factor 2-mediated signaling and metabolic associated fatty liver disease [J]. World J Gastroenterol. 2022;28(48):6909–6921. doi:10.3748/wjg.v28.i48.6909
110. Tamilmani P, Uddandrao V V S, Chandrasekaran P, et al. Linalool attenuates lipid accumulation and oxidative stress in metabolic dysfunction-associated steatotic liver disease via Sirt1/Akt/PPRA-α/AMPK and Nrf-2/HO-1 signaling pathways [J]. Clinics and Research in Hepatology and Gastroenterology. 2023;47(10):102231. doi:10.1016/j.clinre.2023.102231
111. An Q, N RJ, Li X, et al. Recent updates on bioactive properties of linalool [J]. Food Funct. 2021;12(21):10370–10389. doi:10.1039/D1FO02120F
112. Li Y, Yang M, Lin H, et al. Limonin alleviates non-alcoholic fatty liver disease by reducing lipid accumulation, suppressing inflammation and oxidative stress [J]. Front Pharmacol. 2021;12:801730. doi:10.3389/fphar.2021.801730
113. Gao G, Xie Z, W LE, et al. Dehydroabietic acid improves nonalcoholic fatty liver disease through activating the Keap1/Nrf2-ARE signaling pathway to reduce ferroptosis [J]. J Nat Med. 2021;75(3):540–552. doi:10.1007/s11418-021-01491-4
114. E FA, Abdo W, Osman A, et al. Betulin prevents high fat diet-induced non-alcoholic fatty liver disease by mitigating oxidative stress and upregulating Nrf2 and SIRT1 in rats [J]. Life Sci. 2023;322:121688. doi:10.1016/j.lfs.2023.121688
115. Fan J, Chen Q, Wei L, et al. Asiatic acid ameliorates CC l4-induced liver fibrosis in rats: involvement of Nrf2/ARE, NF-κB/IκBα, and JAK1/STAT3 signaling pathways. Drug Des Devel Ther. 2018;12:3595–3605. doi:10.2147/DDDT.S179876
116. Mushtaq Z, Imran M, Hussain M. Asiatic acid: a review on its polypharmacological properties and therapeutic potential against various Maladies [J]. INT J FOOD PROP. 2023;26:1244–1263. doi:10.1080/10942912.2023.2209702
117. Zhang C, Li L, Hou S, et al. Astragaloside IV inhibits hepatocellular carcinoma by continually suppressing the development of fibrosis and regulating pSmad3C/3L and Nrf2/HO-1 pathways [J]. J Ethnopharmacol. 2021;279:114350. doi:10.1016/j.jep.2021.114350
118. Fang Gong Y, Hou S, C XJ, et al. Amelioratory effects of astragaloside IV on hepatocarcinogenesis via Nrf2-mediated pSmad3C/3L transformation [J]. Phytomedicine. 2023;117:154903. doi:10.1016/j.phymed.2023.154903
119. Shao M, Kuang Z, Wang W, et al. Aucubin exerts anticancer activity in breast cancer and regulates intestinal microbiota [J]. eCAM. 2022;2022:4534411. doi:10.1155/2022/4534411
120. Shen B, Feng H, Cheng J, et al. Geniposide alleviates non-alcohol fatty liver disease via regulating Nrf2/AMPK/mTOR signalling pathways [J]. J Cell & Mol Med. 2020;24(9):5097–5108. doi:10.1111/jcmm.15139
121. El Menyiy N, Elouafy Y, Moubachir R, et al. Chemistry, biological activities, and pharmacological properties of gastrodin: mechanism insights [J]. Chem Biodive. 2024;21(6):e202400402. doi:10.1002/cbdv.202400402
122. Alam F, Badruddeen Kharya A, Juber A, Naringin M. Sources, chemistry, toxicity, pharmacokinetics, pharmacological evidences, molecular docking and cell line study [J]. Res J Pharm Technol. 2020;13(5):2507–2515.
123. Li J, Wang T, Liu P, et al. Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD [J]. Food Funct. 2021;12(9):3898–3918. doi:10.1039/D0FO02736G
124. D LX, Y CZ, S YJ, et al. [Effect of total flavones of hawthorn leafonon expression of COX-2/Nrf2 in liver of rats with nonalcoholic steatohepatitis]. Zhongguo Zhong Yao Za Zhi. 2016;41(4):711–715. doi:10.4268/cjcmm20160428
125. G XH, B ZB, Q WZ, et al. Treatment with baicalein attenuates methionine-choline deficient diet-induced non-alcoholic steatohepatitis in rats [J]. Eur J Pharmacol. 2014;738:310–318. doi:10.1016/j.ejphar.2014.06.006
126. Shi H, Qiao F, Lu W, et al. Baicalin improved hepatic injury of NASH by regulating NRF2/HO-1/NRLP3 pathway [J]. Eur J Pharmacol. 2022;934:175270. doi:10.1016/j.ejphar.2022.175270
127. Zhang X, Ji R, Sun H, et al. Scutellarin ameliorates nonalcoholic fatty liver disease through the PPARγ/PGC-1α-Nrf2 pathway [J]. Free Radical Research. 2018;52(2):198–211. doi:10.1080/10715762.2017.1422602
128. Fan H, Ma X, Lin P, et al. Scutellarin Prevents Nonalcoholic Fatty Liver Disease (NAFLD) and hyperlipidemia via PI3K/AKT-Dependent activation of nuclear factor (Erythroid-Derived 2)-Like 2 (Nrf2) in Rats [J]. Medical Science. 2017;23:5599–5612. doi:10.12659/MSM.907530
129. Jiang G, Chen D, Li W, et al. Effects of wogonoside on the inflammatory response and oxidative stress in mice with nonalcoholic fatty liver disease [J]. Pharm Biol. 2020;58(1):1177–1183. doi:10.1080/13880209.2020.1845747
130. Xu Q, Fan Y, J LJ, et al. Aloin protects mice from diet-induced non-alcoholic steatohepatitis via activation of Nrf2/HO-1 signaling [J]. Food Funct. 2021;12(2):696–705. doi:10.1039/D0FO02684K
131. Li B, Wang R, Wang L, et al. Capillin protects against non-alcoholic steatohepatitis through suppressing NLRP3 inflammasome activation and oxidative stress [J]. Immuno and Immunotoxicol. 2021;43(6):778–789. doi:10.1080/08923973.2021.1984520
132. Chen Q, Zhang H, Cao Y, et al. Schisandrin B attenuates CCl(4)-induced liver fibrosis in rats by regulation of Nrf2-ARE and TGF-β/Smad signaling pathways [J]. Drug Des Devel Ther. 2017;11:2179–2191. doi:10.2147/DDDT.S137507
133. I NM, Zhu S, Chen C, et al. A comprehensive review on schisandrin B and its biological properties [J]. Oxid Med Cell Longev. 2020;2020:2172740. doi:10.1155/2020/2172740
134. H WT, W WP, Y LT, et al. Antioxidant properties of red raspberry extract alleviate hepatic fibrosis via inducing apoptosis and transdifferentiation of activated hepatic stellate cells [J]. Biomed Pharmacothe. 2021;144:112284. doi:10.1016/j.biopha.2021.112284
135. P LJ, Gao Y, F CS, et al. Nrf2 pathway activation contributes to anti-fibrosis effects of ginsenoside Rg1 in a rat model of alcohol- and CCl4-induced hepatic fibrosis [J]. Acta Pharmacol. Sin. 2014;35(8):1031–1044. doi:10.1038/aps.2014.41
136. Wang R, Wang J, Song F, et al. Tanshinol ameliorates CCl4-induced liver fibrosis in rats through the regulation of Nrf2/HO-1 and NF-κB/IκBα signaling pathway. Drug Des Devel Ther. 2018;12:1281–1292. doi:10.2147/DDDT.S159546
137. Li S, Wang R, Song F, et al. Salvianolic acid A suppresses CCl(4)-induced liver fibrosis through regulating the Nrf2/HO-1, NF-κB/IκBα, p38 MAPK, and JAK1/STAT3 signaling pathways [J]. Drug Chem Toxicol. 2023;46(2):304–313. doi:10.1080/01480545.2022.2028822
138. Geng Y, N FK, de Meijer V E, et al. How does hepatic lipid accumulation lead to lipotoxicity in non-alcoholic fatty liver disease? Hepatol Internat. 2021;15(1):21–35. doi:10.1007/s12072-020-10121-2
139. Yasmin T, M RM, Khan F, et al. Metformin treatment reverses high fat diet- induced non-alcoholic fatty liver diseases and dyslipidemia by stimulating multiple antioxidant and anti-inflammatory pathways [J]. Biochem. Biophys Rep. 2021;28:101168. doi:10.1016/j.bbrep.2021.101168
140. Chenxu G, Minxuan X, Yuting Q, et al. Loss of RIP3 initiates annihilation of high-fat diet initialized nonalcoholic hepatosteatosis: a mechanism involving Toll-like receptor 4 and oxidative stress [J]. Free Radic Biol Med. 2019;134:23–41. doi:10.1016/j.freeradbiomed.2018.12.034
141. Ramadori P, Drescher H, Erschfeld S, et al. Hepatocyte-specific Keap1 deletion reduces liver steatosis but not inflammation during non-alcoholic steatohepatitis development [J]. Free Radic Biol Med. 2016;91:114–126. doi:10.1016/j.freeradbiomed.2015.12.014
142. Tanaka Y, M AL, L YR, et al. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet [J]. J Pharmacol Exp Ther. 2008;325(2):655–664. doi:10.1124/jpet.107.135822
143. Shelley K, Articolo A, Luthra R, et al. Clinical characteristics and management of patients with nonalcoholic steatohepatitis in a real-world setting: analysis of the Ipsos NASH therapy monitor database [J]. BMC Gastroenterol. 2023;23(1):160. doi:10.1186/s12876-023-02794-4
144. Gutiérrez-Cuevas J, Lucano-Landeros S, López-Cifuentes D, et al. Epidemiologic, genetic, pathogenic, metabolic, epigenetic aspects involved in NASH-HCC: current therapeutic strategies [J]. Cancers. 2022;15(1). doi:10.3390/cancers15010023
145. Dorairaj V, A SS, Abu N, et al. Nonalcoholic Fatty Liver Disease (NAFLD): pathogenesis and noninvasive diagnosis [J]. Biomedicines. 2021;10(1):15. doi:10.3390/biomedicines10010015
146. Singh S, M AA, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies [J]. Clinical Gastroent Hepatol. 2015;13(4):643. doi:10.1016/j.cgh.2014.04.014
147. Ramos-Tovar E, Muriel P. Free radicals, antioxidants, nuclear factor-E2-related factor-2 and liver damage [J]. J Appl Toxicol. 2020;40(1):151–168. doi:10.1002/jat.3880
148. Bellezza I, Tucci A, Galli F, et al. Inhibition of NF-κB nuclear translocation via HO-1 activation underlies α-tocopheryl succinate toxicity [J]. J Nutr Biochem. 2012;23(12):1583–1591. doi:10.1016/j.jnutbio.2011.10.012
149. Wardyn JD, Ponsford AH, Sanderson CM. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways [J]. Biochem. Soc Trans. 2015;43(4):621–626. doi:10.1042/BST20150014
150. Saha S, Buttari B, Panieri E, et al. An overview of Nrf2 signaling pathway and its role in inflammation [J]. Molecules. 2020;25(22). doi:10.3390/molecules25225474
151. M AS, Luo L, Namani A, et al. Nrf2 signaling pathway: pivotal roles in inflammation [J]. Biochimica et biophysica acta Molecular basis of disease. 2017;1863(2):585–597. doi:10.1016/j.bbadis.2016.11.005
152. Okada K, Warabi E, Sugimoto H, et al. Deletion of Nrf2 leads to rapid progression of steatohepatitis in mice fed atherogenic plus high-fat diet [J]. J Gastroenterol. 2013;48(5):620–632. doi:10.1007/s00535-012-0659-z
153. Wang C, Cui Y, Li C, et al. Nrf2 deletion causes ”benign” simple steatosis to develop into nonalcoholic steatohepatitis in mice fed a high-fat diet [J]. Lipids Health Dis. 2013;12:165. doi:10.1186/1476-511X-12-165
154. Bathish B, Robertson H, F DJ, et al. Nonalcoholic steatohepatitis and mechanisms by which it is ameliorated by activation of the CNC-bZIP transcription factor Nrf2 [J]. Free Radic Biol Med. 2022;188:221–261.
155. Q WR, M NY, Han F, et al. The role of heme oxygenase-1 in non-alcoholic steatohepatitis. Zhonghua Gan Zang Bing Za Zhi. 2010;18(9):680–684. doi:10.3760/cma.j.issn.1007-3418.2010.09.009
156. Y LG, J CW, Y JB, et al. Di’ao Xinxuekang alleviates non-alcoholic steatohepatitis in mice by up-regulating Nrf2/HO-1 signaling pathway. Zhongguo Zhong Yao Za Zhi. 2022;47(9):2491–2499. doi:10.19540/j.cnki.cjcmm.20211115.401
157. Bataller R, Brenner DA. Liver fibrosis [J]. J Clin Invest. 2005;115(2):209–218. doi:10.1172/JCI24282
158. Conde de la Rosa L, Goicoechea L, Torres S, Garcia-Ruiz C, Fernandez-Checa J. Role of oxidative stress in liver disorders. Livers. 2022;2(4). doi:10.3390/livers2040023
159. Galicia-Moreno M, Lucano-Landeros S, C M-RH, et al. Roles of Nrf2 in liver diseases: molecular, pharmacological, and epigenetic aspects [J]. Antioxidants. 2020;9(10):980. doi:10.3390/antiox9100980
160. J YJ, Tao H, Huang C, et al. Nuclear erythroid 2-related factor 2: a novel potential therapeutic target for liver fibrosis [J]. Food Chemi Toxicol. 2013;59:421–427. doi:10.1016/j.fct.2013.06.018
161. Q WR, M NY, J WW, et al. Induction of heme oxygenase-1 protects against nutritional fibrosing steatohepatitis in mice [J]. Lipids Health Dis. 2011;10:31. doi:10.1186/1476-511X-10-31
162. Khadrawy SM, Mohamed HM, Mahmoud AM. Mesenchymal stem cells ameliorate oxidative stress, inflammation, and hepatic fibrosis via Nrf2/HO-1 signaling pathway in rats [J]. Environ Sci Pollut Res Int. 2021;28(2):2019–2030. doi:10.1007/s11356-020-10637-y
163. Hu L, Tian K, Zhang T, et al. Cyanate induces oxidative stress injury and abnormal lipid metabolism in liver through Nrf2/HO-1. Molecules. 2019;24(18):3231. doi:10.3390/molecules24183231
164. J MP, Chowdhry S, Sharma RS, et al. Susceptibility of Nrf2-null mice to steatohepatitis and cirrhosis upon consumption of a high-fat diet is associated with oxidative stress, perturbation of the unfolded protein response, and disturbance in the expression of metabolic enzymes but not with insulin resistance [J]. Mol Cell Biol. 2014;34(17):3305–3320. doi:10.1128/MCB.00677-14
165. Wu T, Zhao F, Gao B, et al. Hrd1 suppresses Nrf2-mediated cellular protection during liver cirrhosis [J]. Genes Dev. 2014;28(7):708–722. doi:10.1101/gad.238246.114
166. Zhou J, Zheng Q, Chen Z. The Nrf2 pathway in liver diseases [J]. Front Cell Develop Biol. 2022;10:826204. doi:10.3389/fcell.2022.826204
167. L WC, H LK, E KH, et al. Expression of haem oxygenase in cirrhotic rat liver [J]. J Pathol. 2003;199(3):324–334. doi:10.1002/path.1284
168. Xue H, Guo H, C LY, et al. Heme oxygenase-1 induction by hemin protects liver cells from ischemia/reperfusion injury in cirrhotic rats [J]. World J Gastroenterol. 2007;13(40):5384–5390. doi:10.3748/wjg.v13.i40.5384
169. Yang Y, Xiong L, Li M, et al. Advances in radiotherapy and immunity in hepatocellular carcinoma [J]. J Transl Med. 2023;21(1):526. doi:10.1186/s12967-023-04386-y
170. Li L, Wang Q, He Y, et al. Astragaloside IV suppresses migration and invasion of TGF-β(1)-induced human hepatoma HuH-7 cells by regulating Nrf2/HO-1 and TGF-β(1)/Smad3 pathways [J]. Naunyn-Schmiedeberg’s Arch Pharmacol. 2022;395(4):397–405. doi:10.1007/s00210-021-02199-8
171. Guoyin Z, Hao P, Min L, et al. Antihepatocarcinoma effect of portulaca oleracea L. in Mice by PI3K/Akt/mTOR and Nrf2/HO-1/NF-κB pathway [J]. eCAM. 2017;2017:8231358. doi:10.1155/2017/8231358
172. Zou C, Zou C, Cheng W, et al. Heme oxygenase-1 retards hepatocellular carcinoma progression through the microRNA pathway [J]. Oncology Reports. 2016;36(5):2715–2722. doi:10.3892/or.2016.5056
173. Qin J, He Y, Duan M, et al. Effects of Nuclear Factor-E2-related factor 2/Heme oxygenase 1 on splanchnic hemodynamics in experimental cirrhosis with portal hypertension [J]. Microvascular Res. 2017;111:12–19. doi:10.1016/j.mvr.2016.12.009
174. Liu D, Zhang Y, Wei Y, et al. Activation of AKT pathway by Nrf2/PDGFA feedback loop contributes to HCC progression [J]. Oncotarget. 2016;7(40):65389–65402. doi:10.18632/oncotarget.11700
175. M WM, I HF, Hsu LI, et al. GT-repeat polymorphism in the HO-1 gene promoter is associated with risk of liver cancer: a follow-up study from arseniasis-endemic areas in Taiwan [J]. J Clin Med. 2021;10(7). doi:10.3390/jcm10071489
176. Fernández-Ginés R, A EJ, Escoll M, et al. Specific targeting of the NRF2/β-TrCP axis promotes beneficial effects in NASH [J]. Redox Biol. 2024;69:103027. doi:10.1016/j.redox.2024.103027
177. Mohs A, Otto T, M SK, et al. Hepatocyte-specific NRF2 activation controls fibrogenesis and carcinogenesis in steatohepatitis [J]. J Hepatol. 2021;74(3):638–648. doi:10.1016/j.jhep.2020.09.037
178. Li S, Eguchi N, Lau H, et al. The Role of the Nrf2 Signaling in Obesity and Insulin Resistance [J]. Int J Mol Sci. 2020;21(18):6973.
179. Safdar A, deBeer J, Tarnopolsky MA. Dysfunctional Nrf2-Keap1 redox signaling in skeletal muscle of the sedentary old [J]. Free Radic Biol Med. 2010;49(10):1487–1493. doi:10.1016/j.freeradbiomed.2010.08.010
180. Han X, Ding C, Zhang G, et al. Liraglutide ameliorates obesity-related nonalcoholic fatty liver disease by regulating Sestrin2-mediated Nrf2/HO-1 pathway [J]. Biochem. Biophys. Res. Commun. 2020;525(4):895–901. doi:10.1016/j.bbrc.2020.03.032
181. Nishi T, Yamamoto Y, Yamagishi N, et al. Lansoprazole prevents the progression of liver fibrosis in non-alcoholic steatohepatitis model rats [J]. J Pharm Pharmacol. 2018;70(3):383–392. doi:10.1111/jphp.12870
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Pharmacological Activity of Chemical Compounds of Potato Peel Waste (Solanum tuberosum L.) in vitro: A Scoping Review
Hidayat W, Sufiawati I, Satari MH, Lesmana R, Ichwan S
Journal of Experimental Pharmacology 2024, 16:61-69
Published Date: 3 February 2024