Back to Journals » Journal of Experimental Pharmacology » Volume 17
The Potential Effect of Dapagliflozin and Liraglutide in Attenuating Cardio-Renal Injuries in Diabetic Rats
Authors Albanna AA, Ibrahim DA , Shamsher AM, Al-Shawia MA, Halboup A
Received 11 February 2025
Accepted for publication 24 June 2025
Published 7 July 2025 Volume 2025:17 Pages 467—484
DOI https://doi.org/10.2147/JEP.S522053
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Abdelwahab Omri
Adel Ali Albanna,1 Doa’a Anwar Ibrahim,2 Amani Mohammed Shamsher,3 Mojahed Ali Al-Shawia,4 Abdulsalam Halboup1,5
1Department of Pharmacology, Faculty of Pharmacy, University of Science and Technology, Sana’a, Yemen; 2Department of Clinical Pharmacy and Pharmacy Practice, Faculty of Pharmacy, University of Science and Technology, Sana’a, Yemen; 3Department of Histopathology, University of Science and Technology Hospital, Sana’a, Yemen; 4Department of Biological Science, Faculty of Science, Sana’a University, Sana’a, Yemen; 5Discipline of Clinical Pharmacy, School of Pharmaceutical Science, Universiti Sains, Malaysia, Penang, Malaysia
Correspondence: Doa’a Anwar Ibrahim, Department of Clinical Pharmacy and Pharmacy Practice, University of Science and Technology, Sana’a, Yemen, Email [email protected]
Objective: To evaluate the therapeutic benefits of dapagliflozin and liraglutide as treatments for type 2 diabetes mellitus and their combined effects on T2DM-related complications, specifically cardio-renal injury.
Methods: Thirty rats were randomly allocated into two groups, with the first group serving as the control group, which had 6 rats. The second group was the experimental group, which had 24 rats administered a high-fat diet for four weeks, followed by a single dose of streptozotocin (STZ) to induce diabetes mellitus (DM). This, combined with a high-fat diet, a low dose of STZ was used to cause sub-lethal damage to beta cells. HFD/STZ is an easy method to successfully create a rat model resembling human T2DM, causing insulin resistance, but it does not fully capture the complexity of human T2DM. The experimental group was randomly divided into a positive control group, a liraglutide (0.4 mg/kg, s.c) group, a dapagliflozin (1 mg/kg, orally) group, and a combination of Dapa and lira group, which were administered daily for four weeks. Blood samples were analyzed for glucose, insulin, and cardiac and kidney function markers. Cardiac and kidney tissue were examined to assess redox balance, glutathione (GSH), catalase (CAT), and malondialdehyde (MDA).
Results: Dapa and/or lira administration improved the body weight, lipid profile, cardiac and kidney function markers. Furthermore, all treating groups exhibited restoration of the balance between oxidants and antioxidants. Histological studies also revealed a reduction in cardiorenal tissue injury caused by diabetes. Interestingly, the combined management of Dapa and Lira showed a more beneficial protective effect than individual treatments. This study uniquely explores the simultaneous impact on cardiac and renal systems in a diabetic model, offering novel insights into cardiorenal interaction and the combined therapeutic potential of Dapa and Lira.
Conclusion: These findings suggest that the combination of dapagliflozin and liraglutide provides superior protection against diabetes-induced cardiorenal injury compared to either treatment alone, highlighting their potential as adjunctive therapies in reducing type 2 diabetes mellitus complications.
Keywords: dapagliflozin, liraglutide, cardio-renal injuries
Background
Diabetes mellitus is a major global public health issue, affecting approximately 537 million individuals in 2021 and projected to impact 643 million by 2030 and 783 million by 2045.1
Diabetes mellitus (DM) is a systemic metabolic disorder characterized by chronic hyperglycemia resulting from defects in insulin secretion, action, or both.2,3 The disease is a leading cause of cardiovascular events and diabetic nephropathy, which are the primary contributors to morbidity and mortality in diabetic populations.4–9
T2DM induces persistent hyperglycemia, triggering oxidative stress, chronic inflammation, and endothelial dysfunction, contributing to progressive cardiac and renal tissue injury.10,11 This pathophysiological overlap underscores the importance of therapeutic interventions targeting metabolic control and cardio-renal protection.
Dapagliflozin, a sodium-glucose co-transporter 2 (SGLT2) inhibitor, acts by decreasing renal glucose reabsorption, reducing glomerular hyperfiltration, and modulating hemodynamic and oxidative stress parameters, ultimately offering cardioprotective and nephroprotective benefits. The proposed mechanisms of action are illustrated in Figure 1, demonstrating how SGLT2 inhibition influences key renal and cardiovascular pathways.12–17 Liraglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, enhances glucose-dependent insulin secretion while exhibiting anti-inflammatory and antioxidative properties. It shows beneficial effects on cardiac and renal systems, as shown in Figure 2, which outlines the multifaceted mechanisms contributing to its cardio-renal protection.18–23
 |
Figure 1 Mechanisms of kidney and cardiovascular protection by SGLT2 inhibitors.17 |
 |
Figure 2 Cardio-renal protection mechanisms of GLP-1 receptor agonists.22,23 |
The rationale for combining these agents stems from their mechanistically complementary effects: dapagliflozin primarily improves renal hemodynamics, metabolic parameters, and cardio protection, improving hemodynamic load, reducing inflammation and oxidative stress, while liraglutide acts on inflammatory and oxidative pathways in both cardiac and renal tissues.24,25 Their combination is hypothesized to produce additive or synergistic benefits in attenuating diabetes-induced organ damage.
Therefore, the present study aims to investigate the protective effects of dapagliflozin and liraglutide, alone and in combination, on heart and kidney tissues in a rat model of T2DM induced by a high-fat diet and streptozotocin, focusing on redox biomarkers and histopathological changes.
Materials and Methods
Drugs and Chemicals
The raw material, dapagliflozin, was purchased from the Modern Pharmaceutical factory (Sana’a, Yemen). Liraglutide (VICTOZA, Novo Nordisk, Bagsvaerd, Denmark) and streptozotocin (STZ) were acquired from Sigma Aldrich. All further compounds were acquired from Himedia Company, Sana’a, Yemen.
Experimental Animals and Diets
Thirty adult male albino Wistar rats (10–12 weeks old, weighing 180 ± 20 g) were obtained from Sana’a University’s Animal House, Department of Biology. One week before the start of the treatment, the rats were acclimatized to the lab environment, where six male rats were kept per cage, with a 12-hour light/dark cycle. Before the experiment, the rats were fed a regular diet and water. The rats received precise numbers and marks after being weighed.
In the control group, the rats were fed a regular diet and water, while the other rats in the experimental group were fed a high-fat diet HFD for 4 weeks; treatment was given for 4 weeks; the high-fat diet’s composition was as in previous literature.26,27
Methods
Thirty rats were divided randomly into two groups, a control group (n = 6) and an experimental group (n = 24). After four weeks of feeding a high-fat diet HFD, these rats were overnight fasted and injected with freshly prepared STZ (40mg/ kg IP) single dose,28 dissolved in 0.1 M citrate buffer (pH 4.5).29 The rats in the normal control group received a regular diet, normal saline orally, like the experimental group, and the same dosage of citrate buffer via intraperitoneal injection (1 mL/kg, i.p). The rats in the rest of the groups received drinking water containing 5% sucrose for 48 hours after being injected with STZ. This was done to prevent premature death that could have been caused by insulin release from partially damaged pancreatic islets.30 After 72 hours, fasted rats’ Blood was drawn from a vein in the tail, and blood glucose levels were checked using an Accu-Chek instant glucometer. Rats were determined to have diabetes if their fasting blood glucose (FBG) level was greater than 250 mg/dl, and they were included in the study.31
Experimental Design
The rats confirmed as diabetic were randomly divided into four groups of six in each group—an experimental design according to.32 The following treatment regimen was used on diabetic and non-diabetic rats (Table 1).
 |
Table 1 Animal Treatment Protocol |
Preparation of Citrate Buffer and Streptozotocin (STZ)
A total of 1.5 g of sodium citrate was separately dissolved in 50 mL of distilled water, and 1.05 g of citric acid was dissolved in another 50 mL of distilled water. An amount of 21.5 mL of sodium citrate solution was mixed with 26.7 mL of citric acid solution in a separate sterile beaker, and distilled water was added to make a final volume of 100 mL (pH 4.5).36 One intraperitoneal injection of a single low dose of STZ 40mg/kg, diluted in 0.1 M sodium citrate buffer at pH 4.5 in a volume of 1 mL/kg body weight, was administered to each rat.37
General Phenotypes Observation
Assessment of Body Weight
The alteration in body weight was measured before, during, and at the end of the animal experiment.
Animals’ Blood, Urine, and Organ Sample Collection
Following the study’s conclusion, the animals were transported after the last treatment to be kept separately in metabolic cages for a 24-hour urine collection. The animals were euthanized while under anesthesia with chloroform. For the biological analysis, blood samples were collected, and serum was separated for 15 min at 3000 rpm by centrifugation. The heart and kidneys were collected, saline-washed, and weighed to determine their relative weight. The histological examinations, the kidney and heart were suitably fixed in formalin at 10%.
Determination of Relative Organ Weight
All animals were weighed at the end of the experiment, and the weight of the organ, heart, and kidney of each animal was calculated using the formula below:
Relative weight % = weight of the organ / Rat B.W x 100
Assessment of Body Mass Index (BMI)
The rats’ body lengths (nose-anus), lengths, and weights recorded during and at the end of the experiment were utilized to compute BMI using the following formula: Body mass index (BMI) = body weight (g)/square length (cm2).38
Assessment: Fasting Blood Glucose
Measurements of fasting blood glucose were made with Accu-Chek Instant and its test strip (Roche Diagnostics, Germany), according to.39
Assessment of Insulin Level
ELISA kits (Elecsys Insulin) can be used to measure the levels of serum insulin40 by COBAS e 411 automated analyzers (Roche Company).
Assessment of Serum Lipid Profiles
Including triglyceride, total cholesterol, low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) by Cobas c 501.
Measurement of Serum Levels of Cardiac Biomarkers
Cardiac Troponin I For (Cobas e 411 analyzer) ELISA Kit. Serum creatinine kinase (CK-MB) COBAS INTEGRA 400 plus ELISA kits per the manufacturer’s specifications.
Assessment of Urine Parameters
Urine samples were collected for 24 hours to assess the following parameters: urine volume, creatinine (COBAS INTEGRA 400 plus), proteinuria (Cobas c 702 module), and the creatinine clearance was calculated using the following formula: Creatinine clearance (Ccr) = Urine creatinine X Urine volume (mL)/ Plasma Creatinine X Time (Min) according to.41
Assessment of Biochemical Parameters
Blood samples were used to measure clinical biochemical parameters, including creatinine, serum urea, blood urea nitrogen (BUN) using (COBAS INTEGRA / Cobas c system), and potassium (K), according to the guidelines given by the manufacturer.
Assessment of Oxidative Stress
Cardiac and kidney tissue were homogenized in 10% (w/v) phosphate-buffered saline (PBS) using Omni International’s Omni-125 handheld homogenizer manufactured in Kennesaw, GA, USA. In a 4°C centrifuge, the homogenates were centrifuged at 5000g for 15 minutes. For measuring the glutathione (GSH) by using (Rat Glutathione, GSH ELISA; Biosystems, India), catalase (CAT) by using (Rat Catalase, CAT ELISA; Biosystems, India), and Lipid Peroxide malondialdehyde (MDA) colorimetric method (BIODIGNOSTIC, Egypt).
Histological Examination
Heart and kidneys, after dissection, saline-washed solution, thin sections transformed to formalin-fixed tissues, were dehydrated in a graduated sequence of ethanol before being embedded in paraffin. Following that, at 3μm sections were incised using a microtome (SLEE, Germany) and then stained with hematoxylin-eosin (HE) and Periodic Acid Schiff (PAS); HE and (PAS) stained sections were examined using a digital microscope (Manual Olympus; BX51, JAPAN) at 400X magnification.41
Statistical Analysis
The data were entered and analyzed using GraphPad Prism version 8.4 (GraphPad Software, San Diego, CA, USA). Data were first checked for normality using the Shapiro–Wilk test. Normally distributed data are presented as mean ± standard error of the mean (SEM). One-way ANOVA was used for multiple group comparisons, while two-way ANOVA was performed to assess the main and interaction effects of liraglutide and dapagliflozin treatments. Sidak’s post hoc test was applied to subgroup comparisons. A p-value < 0.05 was considered statistically significant.
Result
Effects of liraglutide and/or Dapagliflozin on Body Weight (BW), Body Mass Index, Heart, and Kidney Relative Weight.
Figure 3a showed that the BW of the diabetic group decreased in comparison to the control group, while showing a significant reduction with treatment groups by liraglutide and/or dapagliflozin as compared to the group with diabetes (p-value = 0.048, 0.0022, 0.0024), respectively. Figure 3b shows that the body mass index of the diabetes rats group exhibited an insignificant increase in body mass index compared to the control group. However, when the liraglutide or dapagliflozin groups or (liraglutide and dapagliflozin combination-treated group) were compared with the diabetic group, there was a significant decrease in the body mass index (p-value = 0.049, 0.0027, and 0.04), respectively. Figure 3c illustrates that the diabetic group showed a non-significant increase in heart-relative weight compared to the control group. Treatment with liraglutide (Lira) and/or dapagliflozin showed a non-significant reduction in heart-to-body weight in diabetic animals. Figure 3d illustrates that the group with diabetes exhibited a significant rise in kidney-relative weight in comparison to the control group p-value = 0.001. Treatment with liraglutide showed a significant reduction in kidney-relative weight in diabetic animals, p-value = 0.039. In comparison, treatment with dapagliflozin or liraglutide combined with dapagliflozin showed a non-significant decrease in kidney-relative weight in diabetic animals.
Effect of Liraglutide and/or Dapagliflozin on FBG and Insulin Levels in Diabetic Rats
Figure 4a shows statistically significant (p value < 0.0001) Elevated blood glucose levels in the group with diabetes mellitus compared to the control group. In contrast, treatment with liraglutide, dapagliflozin, and a combination of liraglutide and dapagliflozin for 4 weeks significantly reduced blood glucose levels compared to the diabetic group (p value = 0.0004, < 0.0001, < 0.0001), respectively. Figure 4b indicates that the insulin level was higher in the diabetic group, but not significantly compared to normal animals. Furthermore, with the administration of dapagliflozin and/or liraglutide, there was a decrease in serum insulin.
Effects of Liraglutide and/or Dapagliflozin on Troponin I, Creatine Kinase (CK-MB) in Diabetic Rats
The effect of liraglutide and/or dapagliflozin treatment on troponin-I. As illustrated in Figure 5a, the diabetic group showed elevated troponin-I levels compared to the normal control group, with a significant P value < 0.0001. Liraglutide, dapagliflozin, and a combination of dapagliflozin and liraglutide treatment showed a significant reduction in troponin-I in diabetic animals P value = 0.038, < 0.0001, and < 0.0001, respectively. As illustrated in Figure 5b, the diabetic group showed an elevated in Creatine kinase level as compared to the normal control group, with a significant P value = 0.0005. Dapagliflozin and a combination of dapagliflozin and liraglutide treatment significantly reduced Creatinine kinase in the diabetic group, P value = 0.007, 0.0019, respectively.
Effects of Liraglutide and/or Dapagliflozin on Lipid Profile in Diabetic Rats
The effect of liraglutide and/or dapagliflozin treatment on cholesterol, LDL, TG, and HDL illustrated in Figures 6a–d, the group with diabetes exhibited a rise in cholesterol, LDL, TG, and decreased HD levels a significant P value = 0.004, 0.0045, 0.009, 0.03 respectively. Was observed when compared to the normal control group. In contrast, liraglutide treatment showed a significant decrease in LDL, TG, and an increase in HDL p value = 0.035, 0.005, 0.01, respectively. Dapagliflozin treatment showed a significant decrease in cholesterol, and LDL p value = 0.049, 0.038, respectively. Liraglutide and dapagliflozin combination treatment showed a significant reduction in cholesterol, LDL, TG, and an increase of HDL in the diabetic animals, P value = 0.01, 005, 0.001, 0.0019, respectively.
Effects of Liraglutide and/or Dapagliflozin Treatment on Renal Function in Diabetic Rats
Liraglutide and/or dapagliflozin treatment affects serum creatinine, creatinine clearance, serum urea, and blood urea nitrogen (BUN). As illustrated in Figures 7a–d. Compared to the normal control animals, the diabetes group had significantly elevated serum creatinine, serum urea, and blood urea nitrogen levels P value = 0.004, 0.02, 0.0004, respectively. In contrast, liraglutide treatment showed a significant reduction in serum creatinine and BUN p value = 0.049, 0.015, respectively) compared to the DM group. Dapagliflozin treatment showed a significant reduction in serum creatinine as compared to the DM group P value = 0.02. Liraglutide and dapagliflozin combination treatment showed a significant reduction in serum creatinine and BUN compared to the DM group, P value = 0.025, 0.01. Creatinine clearance showed a significant increase with the liraglutide and dapagliflozin combination treatment group compared to the diabetic group, P value = 0.016.
Effects of Liraglutide and/or Dapagliflozin Treatment on Urine Biomarkers in Diabetic Rats
Figures 8a–c illustrate the effect of liraglutide and/or dapagliflozin treatment on urine volume, creatinine, and protein. The diabetic group showed a non-significant increase in urine volume compared to the normal control animals. In contrast, a significant increase in protein in urine p value = 0.002 and a significant decrease in urine creatinine p value = 0.047 compared to the normal control group. In comparison, liraglutide and /or dapagliflozin treatment showed a non-significant reduction in urine volume and urine creatinine, while Liraglutide, dapagliflozin, and a combination of dapagliflozin and liraglutide treatment showed a significant reduction in protein in urine (p value = 0.04, 0.01, 0.004), respectively.
Effects of Liraglutide and/or Dapagliflozin Treatment on Oxidative Stress Markers in Heart Tissue
Figures 9a–c illustrate the effect of liraglutide and/or dapagliflozin treatment on oxidative stress markers catalase (CAT), glutathione (GSH), and malondialdehyde (MDA) in heart tissue. The diabetic group showed a significant reduction in CAT, GSH, and an increase in MDA (P value = 0.01, 0.0009, and 0.002, respectively, compared to the control group. In addition, the effect of Liraglutide, dapagliflozin, and a combination of dapagliflozin and liraglutide treatment showed a significant increase in catalase (CAT) in heart tissue, P value = 0.03, 0.01, 0.001, respectively. Dapagliflozin and a combination of liraglutide and dapagliflozin treatment showed a significant increase in glutathione (GSH) in heart tissue, P value = 0.006, 0.001, respectively. While Liraglutide, dapagliflozin, and a combination of dapagliflozin and liraglutide treatment showed a significant decrease in MDA in heart tissue (P value = 0.03, 0.01, and 0.001), respectively.
Effects of Liraglutide and/or Dapagliflozin Treatment on Oxidative Stress Markers in Kidney Tissue
As illustrated in Figures 10a–c, the effect of liraglutide and/or dapagliflozin treatment on oxidative stress markers catalase (CAT), glutathione (GSH), and malondialdehyde (MDA) in kidney tissue. The diabetic group showed a statistically significant reduction in CAT, GSH, and an increase in MDA (P value = 0.02, 0.0005, and < 0.0001), respectively, compared to the control group. Liraglutide, dapagliflozin, and a combination of dapagliflozin and liraglutide treatment showed a significant increase in CAT of kidney tissue, P value = 0.04, 0.04, 0.01, respectively, compared to the diabetic group. Dapagliflozin and a combination of liraglutide and dapagliflozin treatment showed a significant increase in GSH in kidney tissue, P value = 0.03, 0.004, respectively. Liraglutide, dapagliflozin, and a combination of dapagliflozin and liraglutide treatment showed a significant decrease in MDA in kidney tissue levels (p-value = 0.04, 0.009, and 0.0001), respectively, compared to the diabetic group.
Effects of Liraglutide (Lira) and/or Dapagliflozin (Dapa) Treatment on Histopathological Changes in Heart Tissue
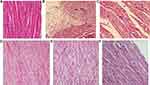
The effect of liraglutide (Lira) and/or dapagliflozin (Dapa) on heart tissue in high-fat diet (HFD) diabetic rats is shown in Figure 11. (A) The normal control rats exhibited a heart muscle with a normal histological structure. (B & C) The diabetic group shows irregular arrangements of swollen myocytes that have distorted nuclei; many myocytes are more eosinophilic with pyknotic nuclei, lysis, disruption, edema, and increased extracellular spaces. There was reduced structural damage in the (E) DM + dapagliflozin and (D) DM + liraglutide treatment groups. The combination therapy group (F) (DM + dapagliflozin + liraglutide) demonstrated nearly full recovery of the normal cardiac architecture.
Effects of Liraglutide (Lira) and/or Dapagliflozin (Dapa) Treatment on Histopathological Changes in Kidney Tissue
The effect of liraglutide (Lira) and/or dapagliflozin (Dapa) on kidney tissue in diabetic rats is shown in Figure 12. That was stained with Periodic Acid-Schiff (PAS) stain, Figure 10, from different experimental groups. (A) The normal control group showed a normal renal cortex with a glomerulus, capsular space, distal and proximal tubules, Bowman’s capsule, and renal corpuscle with an undamaged lining cell brush border. (B and C) Diabetes group (DM) shows small glomeruli with increased mesangial matrix, obliterated capillaries, and wide Bowman’s space. The treatment groups, (D) DM + liraglutide, (E) DM + Dapa, and (F) mixed (DM + Dapa + Lira), all revealed an improvement and almost have similar histology to that of normal.
Two-way ANOVA revealed significant main effects of dapagliflozin and/or liraglutide on all measured cardiometabolic and oxidative stress markers, with dapagliflozin consistently accounting for a larger proportion of the variance. A significant interaction effect was detected only for fasting blood sugar (FBS) (F (1,20) = 7.73, p = 0.0116), suggesting a potential non-additive or synergistic effect. For all other parameters, interaction effects were not statistically significant (p > 0.05), indicating that the treatments acted independently.
Sidak’s post hoc analysis showed that both monotherapies significantly improved outcomes compared to the diabetic control group (p < 0.05). However, combination therapy did not consistently provide additional benefits over monotherapy, supporting a plateau rather than a synergistic effect.
Discussion
The present study used an experimental model in rats that combined a high-fat diet with low-dose STZ (streptozotocin) treatment to induce T2DM rat models, to mimic insulin resistance and partial pancreatic β-cell dysfunction.42 One of the early physiological indicators assessed was body weight, which significantly decreased in diabetic rats compared to healthy controls. This finding is consistent with previous studies, where weight loss in T2DM models has been linked to protein catabolism, muscle wasting, fat breakdown due to insulin deficiency, and osmotic diuresis.43–45 In this study, animals treated with dapagliflozin and liraglutide experienced a more significant reduction in weight compared to diabetic animals. Dapagliflozin, an SGLT2 inhibitor, works by inhibiting the reabsorption of approximately 90% of glucose in the proximal convoluted tubule (PCT), leading to a significant loss in fat mass and visceral adipose tissue.46,47 Liraglutide GLP-1 analog that promotes weight loss through appetite reduction, despite its effects on insulin secretion.48
After 8 weeks of treatment, diabetic rats exhibited elevated fasting blood glucose (FBG) levels, increased cardiac enzymes, dyslipidemia, impaired renal function markers, and oxidative stress. These findings collectively confirmed the successful establishment of the T2DM model and the associated metabolic and organ dysfunction. Elevated FBG with relative hyperinsulinemia observed in the diabetic group reflects insulin resistance, as similarly reported in previous studies.32,49–51
The administration of dapagliflozin led to a greater reduction in blood glucose levels compared to liraglutide monotherapy.32,33 However, the combination of Dapa and Lira produced a more significant decrease in blood glucose compared to either treatment alone. Probably due to improved beta cell function and higher urine glucose excretion.52
The release of cytosolic cardiac enzymes and the potential loss of cardiomyocyte integrity could be due to increased cardiac enzymes in DM group. Other investigations have shown similar findings.33,53 Notably, dapagliflozin exhibited a more potent cardioprotective effect compared to liraglutide, while both dapagliflozin and liraglutide produced superior improvements in cardiac enzymes and myocardial morphology compared to either treatment alone, consistent with previous studies.32,33
Lipid profile analysis revealed a significant increase in total cholesterol, LDL, and triglycerides, along with a decrease in HDL in diabetic rats hallmarks of diabetic dyslipidemia.54 These alterations are attributed to chronic hyperglycemia and insulin resistance disrupting lipid metabolism.55 The results of the current study indicate that dapagliflozin administration improves lipid profiles, similar to previous studies.56–58 However, administration of liraglutide in diabetic rats reduced blood triglyceride, total cholesterol levels, and LDL and increased HDL levels, consistent with previous studies.59,60
Type 2 diabetes with uncontrolled hyperglycemia causes hemodynamic dysregulation and nephron functional abnormalities, which lead to kidney injury. Consequently, there is an elevation in the permeability of plasma proteins across the glomerular filtration barrier, resulting in an augmented excretion of proteins in the urine. Persistent elevation of blood glucose levels generates reactive oxygen species (ROS), and oxidative stress is among the fundamental mechanisms driving the development of nephrotoxicity and diabetic nephropathy.61 Dapagliflozin therapy significantly improved renal function parameters, reducing proteinuria and enhancing creatinine clearance, in accordance with prior studies.58,62 Similarly, liraglutide showed beneficial effects on renal parameters, decreasing serum creatinine and BUN while reducing proteinuria.63–65 The more favorable outcomes in renal markers reported by El-Sherbiny et al compared to our findings may be attributed to the longer treatment duration (8 weeks) employed in their protocol.65
Oxidative stress markers were also significantly altered in diabetic rats, with increased malondialdehyde (MDA) levels and decreased glutathione (GSH) and catalase (CAT) activity in cardiac and renal tissues. These findings reaffirm earlier observations regarding the role of oxidative damage in T2DM-related complications.66,67
In the current study involving the renal tissue of diabetic rats, therapy with dapagliflozin and/or liraglutide resulted in increased concentrations of GSH and CAT while reducing the concentration of MDA. Previous studies on Dapa treatment that support the reduction of oxidative renal tissue injury and are effective in lowering blood glucose levels align with our findings in the current study.34 Various experimental models have reported that Lira exhibits antioxidant properties in diabetic nephropathy.68 The combination of Dapa and Lira treatments had an additive effect in reducing oxidative injury caused by DM and its associated renal complications. Both drugs effectively decreased MDA (malondialdehyde) levels and restored glutathione and catalase activity in the affected tissues, with our findings in the current study and this agrees with a prior study.65
Mechanistically, chronic hyperglycemia contributes to mitochondrial overproduction of reactive oxygen species (ROS), resulting in oxidative damage to lipids, proteins, and DNA, ultimately impairing insulin signaling pathways such as IRS-1/PI3K/Akt.69–72 The observed therapeutic benefits of dapagliflozin and liraglutide may be partially attributed to their roles in modulating oxidative stress and restoring insulin signaling, thereby reducing inflammation and preserving tissue function.16,20
Both Dapa and Lira demonstrate substantial protective effects against cardio-renal injury induced by diabetes mellitus (DM). Consequently, these drugs hold promise as a prospective therapy strategy for individuals with diabetes to prevent the development of long-term complications. Notably, the combination of both medications exhibited an additive effect, further enhancing the cardiovascular protection observed.
Limitations
This study had several limitations. First, regarding the duration, it was conducted for four weeks, as in some previous studies. Second, the animal diabetic model was used in this study to cause sub-lethal damage to beta cells. HFD/STZ is an easy method to successfully create a rat model resembling human T2DM, causing insulin resistance, but it does not fully capture the complexity of human T2DM. Third, the precise mechanistic protective effect of the tested combination had no clear explanation. Future studies should include extended treatment periods, human clinical trials, and molecular pathway analyses to validate and expand upon the outcomes of the current study.
Conclusions and Recommendations
Dapagliflozin and liraglutide demonstrated significant therapeutic benefits in treating type 2 diabetes mellitus and mitigating T2DM-related complications, particularly cardio-renal injuries. Administration of Dapa and/or Lira led to notable improvements in cardiac and kidney function markers, indicating their efficacy in managing diabetes-related organ damage. The treatments restored the balance between oxidants and antioxidants in the body, as evidenced by changes in redox parameters such as malondialdehyde (MDA), glutathione (GSH), and catalase (CAT).
Histological examinations revealed a reduction in cardiorenal tissue injury caused by diabetes, suggesting that Dapa and Lira have protective effects on these vital organs.
The combined treatment of Dapa and Lira exhibited an additive and more favorable protective effect compared to individual treatments, indicating a potential for enhanced therapeutic outcomes with combination therapy. The study’s unique contribution lies in its integrated cardiorenal assessment, which provides a novel perspective on combination therapy effects and highlights its translational relevance.
However, it is important to note that this study was conducted in a preclinical animal model with a limited duration. Further long-term and clinical studies are warranted to evaluate the sustainability and safety.
We recommend conducting long-term studies to evaluate the sustainability of the therapeutic effects of Dapagliflozin and Liraglutide over extended periods, to determine the optimal dosage combination, and to monitor safety under clinical conditions.
Ethical Approval
Approval from the University of Science & Technology; the Ethics Committee for the Care and Use of Laboratory Animals obtained an ethics approval number (1445/002/UREC/UST) which performed in accordance to The Guide for the Care and Use of Laboratory Animals (NRC 2011; eighth edition) (https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf).73
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Federation I. IDF Diabetes Atlas, tenth. International Diabetes. 2021.
2. Association AD. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl. 1):S62–S69. Erratum in: Diabetes Care. 2010;33:e57.
3. Kerner W, Brückel J. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes. 2014;122(07):384–386. doi:10.1055/s-0034-1366278
4. Skyler JS, Bakris GL, Bonifacio E, et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. 2017;66(2):241–255. doi:10.2337/db16-0806
5. Wei J, Tian J, Tang C, et al. The influence of different types of diabetes on vascular complications. J Diabetes Res. 2022;2022:1–12. doi:10.1155/2022/3448618
6. De Rosa S, Arcidiacono B, Chiefari E, Brunetti A, Indolfi C, Foti DP. Type 2 diabetes mellitus and cardiovascular disease: genetic and epigenetic links. Front Endocrinol. 2018;9:2. doi:10.3389/fendo.2018.00002
7. Umanath K, Lewis JB. Update on diabetic nephropathy: core curriculum 2018. Am J Kidney Dis. 2018;71(6):884–895. doi:10.1053/j.ajkd.2017.10.026
8. Dregan A, Chowienczyk P, Molokhia M. Cardiovascular and type 2 diabetes morbidity and all-cause mortality among diverse chronic inflammatory disorders. Heart. 2017;103(23):1867–1873. doi:10.1136/heartjnl-2017-311214
9. Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137–188. doi:10.1152/physrev.00045.2011
10. Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. 2019;11(3):45.
11. Bhatti JS, Sehrawat A, Mishra J, et al. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: current therapeutics strategies and future perspectives. Free Radic Biol Med. 2022;184:114–134. doi:10.1016/j.freeradbiomed.2022.03.019
12. Salvatore T, Galiero R, Caturano A, et al. An overview of the cardiorenal protective mechanisms of SGLT2 inhibitors. Int J Mol Sci. 2022;23(7):3651. doi:10.3390/ijms23073651
13. Nabrdalik-Leśniak D, Nabrdalik K, Irlik K, et al. The influence of SGLT2 inhibitors on oxidative stress in heart failure and chronic kidney disease in patients with type 2 diabetes. Endokrynologia Polska. 2023;74(4):349–362.
14. Verma S, McMurray JJ. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108–2117. doi:10.1007/s00125-018-4670-7
15. Kim NH, Kim NH. Renoprotective mechanism of sodium-glucose cotransporter 2 inhibitors: focusing on renal hemodynamics. Diabet Metabol J. 2022;46(4):543–551. doi:10.4093/dmj.2022.0209
16. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi:10.1056/NEJMoa1812389
17. Liu H, Sridhar VS, Boulet J, et al. Cardiorenal protection with SGLT2 inhibitors in patients with diabetes mellitus: from biomarkers to clinical outcomes in heart failure and diabetic kidney disease. Metabolism. 2022;126:154918. doi:10.1016/j.metabol.2021.154918
18. Bulum T. Nephroprotective properties of the glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonists. Biomedicines. 2022;10(10):2586. doi:10.3390/biomedicines10102586
19. Winiarska A, Knysak M, Nabrdalik K, Gumprecht J, Stompór T. Inflammation and oxidative stress in diabetic kidney disease: the targets for SGLT2 inhibitors and GLP-1 receptor agonists. Int J Mol Sci. 2021;22(19):10822. doi:10.3390/ijms221910822
20. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi:10.1056/NEJMoa1603827
21. Alobaid SM, Alshahrani RM, Alonazi AS, et al. Liraglutide attenuates diabetic cardiomyopathy via the ILK/PI3K/AKT/PTEN signaling pathway in rats with streptozotocin-induced type 2 diabetes mellitus. Pharmaceuticals. 2024;17(3):374. doi:10.3390/ph17030374
22. Kang YM, Jung CH. Cardiovascular effects of glucagon-like peptide-1 receptor agonists. Endocrinol Metab. 2016;31(2):258. doi:10.3803/EnM.2016.31.2.258
23. Kawanami D, Takashi Y. GLP-1 receptor agonists in diabetic kidney disease: from clinical outcomes to mechanisms. Front Pharmacol. 2020;11:967. doi:10.3389/fphar.2020.00967
24. DeFronzo RA. Combination therapy with GLP‐1 receptor agonist and SGLT2 inhibitor. Diabetes Obesity Metab. 2017;19(10):1353–1362. doi:10.1111/dom.12982
25. Hou Y-C, Zheng C-M, Yen T-H, Lu K-C. Molecular mechanisms of SGLT2 inhibitor on cardiorenal protection. Int J Mol Sci. 2020;21(21):7833. doi:10.3390/ijms21217833
26. Srinivasan K, Viswanad B, Asrat L, Kaul C, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52(4):313–320. doi:10.1016/j.phrs.2005.05.004
27. Gheibi S, Kashfi K, Ghasemi A. A practical guide for induction of type-2 diabetes in rat: incorporating a high-fat diet and streptozotocin. Biomed Pharmacother. 2017;95:605–613. doi:10.1016/j.biopha.2017.08.098
28. Aydın AF, Bingül İ, Küçükgergin C, Doğan‐Ekici I, Doğru Abbasoğlu S, Uysal M. Carnosine decreased oxidation and glycation products in serum and liver of high‐fat diet and low‐dose streptozotocin‐induced diabetic rats. Int J Exp Pathol. 2017;98(5):278–288. doi:10.1111/iep.12252
29. Miaffo D, Ntchapda F, Mahamad TA, Maidadi B, Kamanyi A. Hypoglycemic, antidyslipidemic and antioxydant effects of Vitellaria paradoxa barks extract on high-fat diet and streptozotocin-induced type 2 diabetes rats. Metab Open. 2021;9:100071. doi:10.1016/j.metop.2020.100071
30. Ko CY, Lin RH, Lo YM, et al. Effect of Ruellia tuberosa L. on aorta endothelial damage‐associated factors in high‐fat diet and streptozotocin‐induced type 2 diabetic rats. Food sci nutr. 2019;7(11):3742–3750. doi:10.1002/fsn3.1233
31. Ghelani H, Razmovski‐Naumovski V, Nammi S. Chronic treatment of (R)‐α‐lipoic acid reduces blood glucose and lipid levels in high‐fat diet and low‐dose streptozotocin‐induced metabolic syndrome and type 2 diabetes in Sprague‐Dawley rats. Pharmacol Res Perspectives. 2017;5(3):e00306. doi:10.1002/prp2.306
32. Hussein AM, Eid EA, Taha M, Elshazli RM, Bedir RF, Lashin LS. Comparative study of the effects of GLP1 analog and SGLT2 inhibitor against diabetic cardiomyopathy in type 2 diabetic rats: possible underlying mechanisms. Biomedicines. 2020;8(3):43. doi:10.3390/biomedicines8030043
33. El-Shafey M, El-Agawy MSE-D, Eldosoky M, et al. Role of dapagliflozin and liraglutide on diabetes-induced cardiomyopathy in rats: implication of oxidative stress, inflammation, and apoptosis. Front Endocrinol. 2022;13:862394. doi:10.3389/fendo.2022.862394
34. Oraby MA, El-Yamany MF, Safar MM, Assaf N, Ghoneim HA. Dapagliflozin attenuates early markers of diabetic nephropathy in fructose-streptozotocin-induced diabetes in rats. Biomed Pharmacother. 2019;109:910–920. doi:10.1016/j.biopha.2018.10.100
35. Jaikumkao K, Pongchaidecha A, Chueakula N, et al. a SGLT2 inhibitor, slows the progression of renal complications through the suppression of renal inflammation, ER stress, and apoptosis in pre-diabetic rats. Diabetes Obes Metab. 2018;20:2617–2626. doi:10.1111/dom.13441
36. Gomori G. [16] Preparation of buffers for use in enzyme studies. 1955.
37. Stalin A, Irudayaraj SS, Gandhi GR, Balakrishna K, Ignacimuthu S, Al-Dhabi NA. Hypoglycemic activity of 6-bromoembelin and vilangin in high-fat diet fed-streptozotocin-induced type 2 diabetic rats and molecular docking studies. Life Sci. 2016;153:100–117. doi:10.1016/j.lfs.2016.04.016
38. Novelli E, Diniz Y, Galhardi C, et al. Anthropometrical parameters and markers of obesity in rats. Lab Animals. 2007;41(1):111–119. doi:10.1258/002367707779399518
39. Muzaffar H, Qamar I, Bashir M, Jabeen F, Irfan S, Anwar H. Gymnema sylvestre supplementation restores normoglycemia, corrects dyslipidemia, and transcriptionally modulates pancreatic and hepatic gene expression in alloxan-induced hyperglycemic rats. Metabolites. 2023;13(4):516. doi:10.3390/metabo13040516
40. Reeves W. Insulin antibody determination: theoretical and practical considerations. Diabetologia. 1983;24:399–403. doi:10.1007/BF00257336
41. Garud MS, Kulkarni YA. Eugenol ameliorates renal damage in streptozotocin‐induced diabetic rats. Flavour Fragrance J. 2017;32(1):54–62. doi:10.1002/ffj.3357
42. X-x G, Wang Y, Wang K, B-p J, Zhou F. Stability of a type 2 diabetes rat model induced by high-fat diet feeding with low-dose streptozotocin injection. J Zhejiang Univ Sci B. 2018;19(7):559. doi:10.1631/jzus.B1700254
43. Thomson SC, Rieg T, Miracle C, et al. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol. 2012;302(1):R75–R83. doi:10.1152/ajpregu.00357.2011
44. Rebolledo-Solleiro D, Fernández-Guasti A. Influence of sex and estrous cycle on blood glucose levels, body weight gain, and depressive-like behavior in streptozotocin-induced diabetic rats. Physiol Behav. 2018;194:560–567. doi:10.1016/j.physbeh.2018.06.033
45. Sundaram R, Nandhakumar E, Haseena Banu H. Hesperidin, a citrus flavonoid ameliorates hyperglycemia by regulating key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Toxicol Mech Methods. 2019;29(9):644–653. doi:10.1080/15376516.2019.1646370
46. Han Y, Li Y-F, Ye C-W, et al. Effects of dapagliflozin on body weight in patients with type 2 diabetes mellitus: evidence‑based practice. Exp Ther Med. 2024;27(4):1–10. doi:10.3892/etm.2024.12461
47. Bolinder J, Ljunggren Ö, Johansson L, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obesity Metab. 2014;16(2):159–169. doi:10.1111/dom.12189
48. Szydlarska D, Jakubowska A, Owoc J, Wierzba W. Evaluation of the effect of liraglutide therapy on body weight and insulin resistance. Medycyna Ogólna i Nauki o Zdrowiu. 2023;29(4):290–292. doi:10.26444/monz/176898
49. Hussein -AE-AM, Omar NM, Sakr H, Elsamanoudy AZ, Shaheen D. Modulation of metabolic and cardiac dysfunctions by insulin sensitizers and angiotensin receptor blocker in rat model of type 2 diabetes mellitus. Can J Physiol Pharmacol. 2011;89(3):216–226. doi:10.1139/Y11-012
50. Naidu PB, Ponmurugan P, Begum MS, et al. Diosgenin reorganises hyperglycaemia and distorted tissue lipid profile in high‐fat diet–streptozotocin‐induced diabetic rats. J Sci Food Agric. 2015;95(15):3177–3182. doi:10.1002/jsfa.7057
51. Taheri Rouhi SZ, Sarker MMR, Rahmat A, Alkahtani SA, Othman F. The effect of pomegranate fresh juice versus pomegranate seed powder on metabolic indices, lipid profile, inflammatory biomarkers, and the histopathology of pancreatic islets of Langerhans in streptozotocin-nicotinamide induced type 2 diabetic Sprague–Dawley rats. BMC Complementary Alternative Med. 2017;17(1):1–13. doi:10.1186/s12906-016-1505-2
52. Bailey CJ, Tahrani AA, Barnett AH. Future glucose-lowering drugs for type 2 diabetes. Lancet Diabetes Endocrinol. 2016;4(4):350–359. doi:10.1016/S2213-8587(15)00462-3
53. El-Sayed N, Mostafa YM, AboGresha NM, Ahmed AA, Mahmoud IZ, El-Sayed NM. Dapagliflozin attenuates diabetic cardiomyopathy through erythropoietin up-regulation of AKT/JAK/MAPK pathways in streptozotocin-induced diabetic rats. Chem Biol Interact. 2021;347:109617. doi:10.1016/j.cbi.2021.109617
54. Krauss RM. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care. 2004;27(6):1496–1504. doi:10.2337/diacare.27.6.1496
55. Hassan SK, El-Sammad NM, Mousa AM, et al. Hypoglycemic and antioxidant activities of Caesalpinia ferrea Martius leaf extract in streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed. 2015;5(6):462–471. doi:10.1016/j.apjtb.2015.03.004
56. Gallwitz B. The cardiovascular benefits associated with the use of sodium-glucose cotransporter 2 inhibitors–real-world data. Eur Endocrinol. 2018;14(1):17. doi:10.17925/EE.2018.14.1.17
57. Saleh S, Hanna G, El-Nabi SH, El-Domiaty H, Shabaan A, Ewida SF. Dapagliflozin, a sodium glucose cotransporter 2 inhibitors, protects cardiovascular function in type-2 diabetic murine model. J Genetics. 2020;99:1–8. doi:10.1007/s12041-020-01196-9
58. Xue S, Li Y-X, Lu -X-X, Tang W. Dapagliflozin can alleviate renal fibrosis in rats with streptozotocin‑induced type 2 diabetes mellitus. Exp Ther Med. 2023;26(6):1–10. doi:10.3892/etm.2023.12271
59. Zhang M, Zhang H, Liu C, et al. Myocardial protective effects of nicorandil on rats with type 2 diabetic cardiomyopathy. Medical Science Monitor Basic Research. 2018;24:141. doi:10.12659/MSMBR.910974
60. Sedky AA. Improvement of cognitive function, glucose and lipid homeostasis and serum osteocalcin levels by liraglutide in diabetic rats. Fundament Clinic Pharmacol. 2021;35(6):989–1003. doi:10.1111/fcp.12664
61. Fakhruddin S, Alanazi W, Jackson KE. Diabetes-induced reactive oxygen species: mechanism of their generation and role in renal injury. J Diabetes Res. 2017;2017. doi:10.1155/2017/8379327
62. Elkazzaz SK, Khodeer DM, El Fayoumi HM, Moustafa YM. Role of sodium glucose cotransporter type 2 inhibitors dapagliflozin on diabetic nephropathy in rats; Inflammation, angiogenesis and apoptosis. Life Sci. 2021;280:119018. doi:10.1016/j.lfs.2021.119018
63. Chen P, Shi X, Xu X, et al. Liraglutide ameliorates early renal injury by the activation of renal FoxO1 in a type 2 diabetic kidney disease rat model. Diabetes Res Clin Pract. 2018;137:173–182. doi:10.1016/j.diabres.2017.09.006
64. Zhao X-Y, Yu -T-T, Liu S, Liu Y-J, Liu JJ, Qin J. Effect of liraglutide on endoplasmic reticulum stress in the renal tissue of type 2 diabetic rats. World J Diab. 2020;11(12):611. doi:10.4239/wjd.v11.i12.611
65. El-Sherbiny M, El-Shafey M, Said E, et al. Dapagliflozin, liraglutide, and their combination attenuate diabetes mellitus-associated hepato-renal injury—insight into oxidative injury/inflammation/apoptosis modulation. Life. 2022;12(5):764. doi:10.3390/life12050764
66. Wilson AJ, Gill EK, Abudalo RA, Edgar KS, Watson CJ, Grieve DJ. Reactive oxygen species signalling in the diabetic heart: emerging prospect for therapeutic targeting. Heart. 2017;293–299. doi:10.1136/heartjnl-2017-311448
67. Xu J, Zhou Q, Xu W, Cai L. Endoplasmic reticulum stress and diabetic cardiomyopathy. Exp Diab Res. 2012;2012:1–12. doi:10.1155/2012/827971
68. Hendarto H, Inoguchi T, Maeda Y, et al. GLP-1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin-induced diabetic rats via protein kinase A-mediated inhibition of renal NAD (P) H oxidases. Metabolism. 2012;61(10):1422–1434. doi:10.1016/j.metabol.2012.03.002
69. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. diabetes. 2005;54(6):1615–1625. doi:10.2337/diabetes.54.6.1615
70. Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–790. doi:10.1038/35008121
71. Lee YS, J-w K, Osborne O, et al. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell. 2014;157(6):1339–1352. doi:10.1016/j.cell.2014.05.012
72. Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7(2):85–96. doi:10.1038/nrm1837
73. National research council of the national academies guide for the care and use of laboratory animals. Washington DC, USA: The National Academies Press; 2011. Available from: https://grants.nih.gov/grants/olaw/guide-for-the-care-and-use-of-laboratory-animals.pdf.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.