Back to Journals » Journal of Inflammation Research » Volume 17
The Potential of Neuregulin 4 as a Novel Biomarker and Therapeutic Agent for Vascular Complications in Type 2 Diabetes Mellitus
Authors Dan X , Li K, Xu J, Yan P
Received 19 August 2024
Accepted for publication 29 October 2024
Published 9 November 2024 Volume 2024:17 Pages 8543—8554
DOI https://doi.org/10.2147/JIR.S492115
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Xiaofang Dan,1– 3 Ke Li,1– 3 Jiali Xu,4 Pijun Yan1– 3,5,6
1Department of Endocrinology and Metabolism, the Affiliated Hospital of Southwest Medical University, Luzhou, People’s Republic of China; 2Metabolic Vascular Disease Key Laboratory of Sichuan Province, Luzhou, People’s Republic of China; 3Sichuan Clinical Research Center for Diabetes and Metabolism, Luzhou, People’s Republic of China; 4Department of Gastroenterology, the Affiliated Hospital of Southwest Medical University, Luzhou, People’s Republic of China; 5Sichuan Clinical Research Center for Nephropathy, Luzhou, People’s Republic of China; 6Cardiovascular and Metabolic Diseases Key Laboratory of Luzhou, Luzhou, People’s Republic of China
Correspondence: Pijun Yan, Email [email protected]
Abstract: Neuregulin 4 (Nrg4), a novel adipokine produced primarily by brown adipose tissue (BAT), has been functionally characterized to exert beneficial effects on modulating energy homeostasis and glucolipid metabolism, and is closely associated with the development and progression of obesity and obesity-associated metabolic diseases, such as type 2 diabetes mellitus (T2DM) and cardiovascular diseases. Recently, there has been a growing focus on the relationship between circulating Nrg4 levels and T2DM-related vascular complications. In this review, we discussed the known and potential roles of Nrg4 in various physiological and pathological processes, and its association with vascular complications in T2DM, in the aim of finding a potential biomarker recommended for the clinical diagnosis, prognosis and follow-up of T2DM patients at high risk of developing vascular complications as well as providing new therapeutic approaches.
Keywords: neuregulin 4, adipokine, diabetes, type 2, vascular complications
Introduction
Type 2 diabetes mellitus (T2DM) is a systemic metabolic disease that seriously affects human health, accounting for approximately 90% of all types of diabetes mellitus, and its prevalence is continually rising worldwide.1 T2DM is linked to both chronic microvascular (diabetic cardiomyopathy, nephropathy, retinopathy, and neuropathy) and macrovascular [coronary artery disease (CAD), peripheral arterial disease (PAD) and stroke] complications, which are the main causes of death and disability in diabetic patients. Considering the morbidity and mortality caused by vascular complications in such population, there is an urgent need to find a reliable and convenient indicator to predict and treat diabetic vascular complications.
Adipose tissue, as a key endocrine organ, is closely related to the pathophysiology of diabetic vascular complications by releasing multiple bioactive substances, known as adipokines. Neuregulin 4 (Nrg4), a recently discovered adipokine, is highly expressed and secreted in brown adipose tissue (BAT) and white adipose tissue (WAT). Numerous studies have showed that Nrg4 plays an important role in regulating neurobiogenesis, glucose and lipid metabolism, insulin sensitivity, inflammation and angiogenesis.2–9 Current research suggests that Nrg4 is significantly decreased in adipose tissue and serums during aging, indicating that Nrg4 may be a potential therapeutic target for the treatment of age-related metabolic diseases such as obesity, T2DM and their vascular complications.6
Recently, Nrg4 as a modulating adipokine, has gained increasing attention for its possibility of application in the treatment of diabetic vascular complications. In this review, we summarize the possible mechanisms underlying the actions of Nrg4 in diabetic vascular complications, hoping to find a potential biomarker of diabetic vascular complications and provide a new therapy strategy for future clinical applications.
Nrg4: An Overview
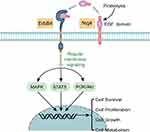
Nrg4, a newly described adipokine, belongs to the neuregulin family of EGF. Nrg4 contains an EGF-like domain that is released in the circulation after proteolysis and acts via autocrine, paracrine, or endocrine mechanisms.10,11 Nrg4 activates intracellular signaling mainly through binding with the erb-b2 receptor tyrosine kinase 4 (ErbB4), thereby exerting its important physiological functions in stimulating cell proliferation, inhibiting cell apoptosis, and regulating glucose and lipid metabolism through phosphatidylinositol-3 kinase/protein kinase B (PI3K/Akt), mitogen-activated protein kinases (MAPK), signal transducer and activator of transcription 5 (STAT5) and other downstream signaling pathways (Figure 1).3,8,12 In liver tissue, Nrg4 inhibits hepatic lipogenesis and stress-induced hepatocyte apoptosis, thus resulting in the prevention of diet-induced nonalcoholic fatty liver disease (NAFLD).13
Nrg4 is highly expressed and secreted in adipose tissue, with the highest level in BAT, followed by WAT, and a relatively low level in other tissues such as liver, heart, brain and skeletal muscle.2,3 Although Nrg4 has the highest expression level in the BAT, its content in BAT is relatively small or even undetectable due to the significant degeneration of BAT in adults. Since white fat is abundant in human, white fat may be an important source of Nrg4 in the human circulation.
Nrg4 expression was significantly induced during adipocytes differentiation, and could be induced by norepinephrine in differentiated brown adipocytes.3 Meanwhile, Nrg4 expression was regulated by cold stimulation, increased only in brown fat tissue after short-term rapid cold exposure, but increased in both brown fat tissue and inguinal white fat tissue after cold acclimation induced by long-term cold exposure.3 The possible reason may be that the sympathetic innervation of BAT is denser and more sensitive to cold stimulation.
Nrg4 and Diabetic Vascular Complications
Although it is well known that Nrg4 plays crucial roles in regulating insulin sensitivity, inflammation, and glucose and metabolism, the relationship between Nrg4 levels and diabetic vascular complications is less clear. In recent years, accumulating evidence shows elevated Nrg4 levels in patients with one or more type 2 diabetic vascular complications [Table 1].
 |
Table 1 Clinical Studies Measuring Circulating Nrg4 Levels in Association with Type 2 Diabetic Vascular Complications |
Nrg4 and Cardiovascular Disease (CVD)
CVD is the leading cause of death and disability among people with diabetes. In recent years, understanding the role of Nrg4 in the cardiovascular system and how it responds to physiological and pathological stress is rapidly evolving. Previous studies have showed that Nrg4 levels were inversely associated with carotid intima-media thickness (CIMT),19 a biomarker of atherosclerosis that could predict cardiovascular events in the general population.20,21 In another study, Nrg4 levels were found to be remarkably decreased in patients with CAD.22 Similarly, Rahimzadeh et al revealed a negative correlation between circulating Nrg4 levels and risk of acute coronary syndrome (ACS).23 Additionally, Nrg4 was identified as a cardioprotective protein since administration of Nrg4 could significantly alleviate experimental myocardial ischemia in mouse model.24 In the study of yang et al, upregulation of Nrg4 gene expression levels could inhibit the proliferation and apoptosis of cardiomyocytes in spontaneous hypertension rats (SHR), and reverse myocardial fibrosis.25 Recently, a case-control study has tested circulating Nrg4 levels in 80 T2DM patients with coronary heart disease (CHD) group versus 77 T2DM patients without CHD controls, and reported that Nrg4 was an independent protective factor for T2DM complicated with CHD.14 Based on the aforementioned researches, we can speculate that circulating Nrg4 might be a new protective adipokine with protective properties against the progression of CAD in T2DM. Indeed, animal studies have showed a vital role of Nrg4/erb-b2 receptor tyrosine kinase 4 (ErbB4) signaling pathway in controlling cardiomyocytes survival, growth, differentiation, and migration.25 However, as a newly discovered adipokine, the exact role of Nrg4 in cardiovascular system is not completely clear, and still needs to be further studied.
Nrg4 and PAD
PAD has been defined as a chronic arterial occlusive disease of the lower extremities due to atherosclerosis.26 The ankle-brachial index (ABI) is a reasonably accurate measurement for the detection of PAD and has been widely used to diagnose lower extremity PAD.27,28 An observational prospective cohort study including 3004 subjects in japan has demonstrated that low ABI is an independent predictor of all-cause death and cardiovascular events both in diabetic and non-diabetic patients.29 Diabetic patients have higher risks for developing PAD, and the prevalence of PAD was significantly higher in elderly diabetics compared with non-diabetics.30 However, to date, only one study consisting of 164 newly diagnosed T2DM patients explored the relationship between Nrg4 and PAD, and reported that circulating Nrg4 levels were neither related to ABI values nor to prevalence of PAD.15 Nevertheless, this study still has some limitations due to its cross-sectional study design, small sample size and different ethnicity restriction. Thus, more well-designed prospective research are urgently needed to confirm this finding.
Nrg4 and Cerebrovascular Disease
Cerebrovascular disease comprises mainly stroke, which is one of the major macrovascular complications of T2DM. It was reported that patients with diabetes had higher risks of developing both ischemic stroke and hemorrhagic stroke compared to those without diabetes.31 Atherosclerosis and thrombosis are the main risk factors for stroke, and patients with carotid atherosclerosis may be more likely to have a stroke due to plaque shedding and vascular stenosis. As we mentioned before, Nrg4 displays the inhibitory effect on inflammation and atherosclerosis,9,12,23 which may provide a link between Nrg4 and cerebrovascular disease. However, up to now, no study has analyzed the association between Nrg4 and cerebrovascular diseases, thus more studies are required to elucidate the exact relationship between Nrg4 and cerebrovascular disease.
Nrg4 and Diabetic Cardiomyopathy
Diabetic cardiomyopathy, a serious and underrecognized diabetic complication, is identified as myocardial structural or functional abnormalities in the absence of CAD, valvular disease, and hypertension.32 Clinically, diabetic cardiomyopathy is characterized initially by myocardial fibrosis, dysfunctional cardiac remodeling, and advancing diastolic dysfunction, later by systolic dysfunction, and ultimately by clinical heart failure.33 Numerous studies have shown that autophagy is essential for maintaining normal cardiac morpholsogy and function.34 In view of this finding, Wang et al proposed that autophagy levels were reduced in mice with type 1 diabetes, and Nrg4 intervention could reactivate autophagy, suggesting that Nrg4 may prevent diabetic cardiomyopathy by regulating autophagy levels through the AMPK/mechanistic target of rapamycin (mTOR) signalling pathway in type 1 diabetic mice.35 In addittion, Nrg4 has been proved to have an direct anti-apoptotic effect on cardiomyocytes through activation of PI3K/Akt signaling pathway.36 However, there is no direct experimental evidence to test the relationship between Nrg4 and diabetic cardiomyopathy in T2DM.
Nrg4 and Diabetic Nephropathy (DN)
DN, also kown as diabetic kidney disease (DKD), is one of the most common diabetic microvascular complications and has become the main cause of end-stage renal disease worldwide. Several mechanisms of DN have been postulated, such as excessive generation of advanced glycosylation end products (AGEs), inflammatory cytokines and oxidative stress.37–39 Among these, oxidative stress is considered as a unifying pathogenic mechanism of diabetic vascular complications. Additionally, tubulointerstitial fibrosis (TIF), characterized by excessive deposition of extracellular matrix (ECM) components,40 always occurs in the early stage of DN. Furthermore, a previous research has indicated that Nrg4 attenuated tubulointerstitial fibrosis and AGEs accumulation through tumor necrosis factor-receptor 1 (TNF-R1) signaling pathway in rat model of DN, suggesting that Nrg4 possessed a therapeutic effect on TIF in DN.41 Kocak et al demonstrated that Nrg4 levels in patients with DN were significantly lower than those with non-DN.17 A similar finding that the levels of Nrg4 in patients with DKD were significantly decreased than that in the healthy control group was also reported in another study.18 Consistent with the above findings, Kralisch et al showed that Nrg4 gene expression levels were decreased in mice with DKD and were independently related to a preserved renal function.42 Recently, Deng et al have found that Nrg4 intervention could attenuate podocyte injury in DN mice, partly by activating the AMPK/mTOR-mediated autophagy signaling pathway.43 However, a cross-sectional study conducted by Yan et al showed no association between circulating Nrg4 and DN.15 These discrepant findings may be partially explained by different study designs, sample size, diabetes duration, medication use and statistical power. Thus, more well-designed studies are required to further validate these fndings.
Nrg4 and Diabetic Peripheral Neuropathy (DPN)
DPN is the main cause of foot ulcer and non-traumatic amputation, which seriously affects diabetic patients’s quality of life and leads to a huge economic burden.16,44 It has been reported that 30% of diabetic patients are complicated with DPN.45 However, the pathogenesis of DPN remains poorly understood. While some studies have demonstrated that IR, dyslipidemia, oxidative stress, inflammation, mitochondrial dysfunction, and microangiopathy may all participate in the occurrence and development of DPN,46–49 its pathogenesis remains to be fully understood. As a member of the neuregulins, Nrg4 may have an important effect in neuronal survival, neurite outgrowth and myelin protection like Nrg1.2,50 Moreover, Nrg4 has been reported to possess neuroprotective and neurotrophic effects, and can promote the outgrowth of neurite and the development of neuronal progenitor stem cells.51–53 A preliminary in vivo study performed by Yan et al has demonstrated decreased Nrg4 levels in newly diagnosed T2DM patients with DPN.16 Similarly, Kocak et al also found that Nrg4 levels in DPN patients were significantly decreased by subgroup analysis and Nrg4 may be a predictive marker of DPN.17 A later study conducted by Yan et al showed a negative association between Nrg4 and vibratory perception threshold (VPT), a reliable marker for early detection of DPN.15 Moreover, they further proposed that decreased circulating Nrg4 levels might result in DPN through a close interaction with 25-hydroxyvitamin D.15 All the above findings consistently suggest that circulating Nrg4 might be a protective factor against DPN.
Nrg4 and Diabetic Retinopathy (DR)
DR is the most common cause of vision loss in working-age adults.54 Recently, DR was defined as a neurovascular disease associated with tissue-specific neurovascular impairment of the retina in diabetic patients. DR, as a common diabetic microvascular complication, may have similarities with other diabetic microvascular complications as we mentioned above in pathogenesis. Hyperglycemic toxicity, inflammation, oxidative stress, and endothelial damage can all cause microvascular injury, if the injury occurs in the retina, it presents as DR. Indeed, several biochemical mechanisms have been proposed to elucidate the pathogenesis of DR, including AGEs accumulation, inflammation, oxidative stress, protein kinase C (PKC) activation, and vascular endothelial growth factor (VEGF).55 Additionally, neuronal abnormalities including neuronal cell death have been demonstrated to be irreversible changes preceding vascular abnormalities in the early stages of DR.56–58 As we previously noted, Nrg4 has neuroprotective and neurotrophic effects,51–53 suggesting that Nrg4 may also have protective effects on DR. However, Kocak et al showed that Nrg4 levels were decreased in patients with DR than non-DR patients, but there was no statistically significant difference.17 Similarly, a later study conducted by Yan et al also showed no statistically significant difference in Nrg4 levels between T2DM patients with and without DR.15 Since the current studies on the relationship between Nrg4 and DR are quite scare and the sample size is limited, the association between Nrg4 and DR is still unclear. Therefore, more evidence is urgently needed to validate these findings.
Mechanisms of Nrg4 in Vascular Complications of T2DM
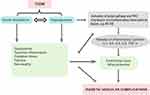
Endothelial dysfunction is a critical and initiating factor in the occurrence and development of diabetic vascular complications,59,60 eventually leading to vascular endothelial injury. The pathogenesis of vascular complications in T2DM is associated with many factors, among which the most important factors are IR and hyperglycaemia (Figure 2). Currently, the precise pathophysiological mechanism of Nrg4 in diabetic vascular complications still remains unclear. Nevertheless, several pathophysiologic mechanisms have been postulated for the role of Nrg4 in diabetic vascular diseases. Here we focus on the description of several known mechanisms that have been demonstrated in various experimental models.
Role of Nrg4 in IR
IR plays a pivotal role in the development and progression of T2DM and its vascular complications. It is generally believed that IR is closely related to chronic inflammation, which requires the activation of the transcription factor nuclear transcription factor-kappa B (NF-kB).61 Insulin stimulates the production of the potent vasodilator nitric oxide (NO) from endothelium by binding to insulin receptors on vascular endothelial cells.62 In IR states, the vasodilator actions of insulin dependent insulin signaling pathways in endothelium are impaired, leading to endothelial dysfunction. Also, the IR state reflects the existence of low-grade inflammation, which may affect vascular endothelium function through inflammatory response, thus jointly leading to angiopathy in T2DM.
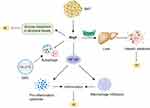
It was revealed that Nrg4 gene transfer improved insulin sensitivity by inhibiting chronic inflammation of white adipose tissue in high-fat diet (HFD)-induced obese mice (Figure 3).9 Additionally, a previous research in mouse models has confirmed that Nrg4 can improve diet-induced IR through activating the STAT5 pathway to prevent de novo lipogenesis in liver.3 In a subsequent study, Chen et al showed that the insulin sensitivity was increased in Nrg4 transgenic mice by promoting the utilization of glucose in peripheral tissues, thereby regulating systemic energy metabolism.63 Consistently, it has been demonstrated that downregulation of Nrg4 expression triggered IR in mice adipocytes, which was likely the result of reduced insulin receptor expression and glucose transporter four (GLUT4) protein levels.64 Moreover, Nrg4 could inhibit inflammation and restore insulin receptor and GLUT4 protein expression.64 In addition, the authors further verified that Nrg4 could reduce autophagy in GLUT4 storage vesicle (GSV) protein to improve glucose metabolism and IR.64 Of note, South et al indicated that Nrg4-ErbB4 signaling could promote insulin secretion by activating PI3K signaling pathway.65 In view of these findings, Nrg4 can improve insulin sensitivity in different ways, raising the possibility that Nrg4 may play a crucial role in diabetic vascular complications due to impaired insulin sensitivity.
Despite Nrg4’s beneficial effects on IR in experimental animal models, it appears that Nrg4 has also adverse effects in human studies. Martínez et al has found a negative correlation between Nrg4 levels and insulin sensitivity in humans,66 contrary to another human study that Nrg4 gene expression was positively associated with insulin sensitivity.67 In addition, they proposed that Nrg4 could attenuate mitochondrial respiration in the human HepG2 cell line but had no effect on the expression of genes related to lipid metabolism.66 These inconsistent findings suggest that there may be other factors influencing Nrg4 level in human. In fact, the authors noted that the lack of protein-based data may lead to the conflicting conclusions.66 To explain these contradictory results, more well-designed research is urgently needed in the future.
Role of Nrg4 in Inflammation
Chronic low-grade inflammation has a strong association with IR in obese objects,68 which could significantly lead to T2DM and its vascular complications. Researchers have reported that the expression of mRNA genes related to hepatic inflammation, including Tnfa, Il1b, Il12b, Nos2, Ccl2, Ccl5, and Adgre1 (F4/80), was significantly upregulated in Nrg4-deficient mice.13 Additionally, a cross-sectional study revealed a negative association between circulating Nrg4 and high-sensitivity C-reactive protein (hs-CRP).69 Consistently, the expression of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), IL-1β, IL-6, IL-8 and IL-9, was also revealed to be negatively associated with Nrg4 levels.64,67,70 Moreover, Ma et al found that Nrg4 overexpression could inhibit chronic inflammation by reducing the expression of macrophage marker monocyte chemotactic protein 1 (MCP-1) gene and increasing the expression of M2 macrophage marker gene Cd163,9 which was in accordance with a subsequent study showing that Nrg4-ErbB4 signaling pathway could stimulate the apoptosis of pro-inflammatory macrophages (M1 macrophage) to ameliorate inflammation in Crohn’s disease.71 In fact, this is probably not the only mechanism by which Nrg4 regulates inflammation. For instance, evidence showed that Nrg4 could enhance adipose tissue angiogenesis to alleviate hypoxia and inflammation in obesity.72 Indeed, Nrg4 gene transfer in mice has been demonstrated to stimulate VEGF expression.63 These findings indicate that Nrg4 might play important roles in protecting against inflammation.
Role of Nrg4 in Oxidative Stress
Oxidative stress often occurs when the concentration of reactive oxygen species (ROS) exceeds that of antioxidant neutralizing species.73 It is generally recognized that increased oxidative stress may be a unifying pathogenic mechanism of diabetic vascular complications. Many of biochemical pathways activated by hyperglycemia, including glucose oxidation, formation of AGEs, and polyol pathways activation, are related to the production of ROS, eventually resulting in the increase of oxidative stress.74,75 Nrg4 gene expression levels were revealed to be negatively regulated by oxidative stress.76 Furthermore, Yan et al found that Nrg4 levels in newly-diagnosed T2DM patients were negatively correlated with 8-iso-prostaglandin F2α (8-iso-PGF2α) and gamma-glutamyl transferase (GGT), two markers of oxidative stress.16 These findings indicate that Nrg4 may be used as a potential oxidative stress marker, and Nrg4 may protect against diabetic vascular complications due to its antioxidant property.
Role of Nrg4 in Atherosclerosis
Atherosclerosis is generally considered an inflammatory response to chronic vascular injury, which is associated with perturbed vascular flow and vascular inflammation mediated by oxidized lipoprotein.77 It is reported that inflammation is a critical risk factor in the development of atherosclerosis and requires the activation of NF-kB.78 According to this concept, Nrg4 may protect against atherosclerotic partly mediated by its anti-inflammatory property as we mentioned above. Indeed, Nrg4 levels have been reported to be inversely associated with CIMT, a biomarker for atherosclerosis and increased angiographic severity of CAD and ACS.22,23 Recently, Shi et al demonstrated that patients with atherosclerosis had significantly lower Nrg4 levels than controls.79 They also observed impaired endothelial function or integrity and an increase of endothelial cells apoptosis in BAT-specific Nrg4 knockout mice.79 Moreover, they further confirmed the important role of Nrg4 in preventing atherosclerosis by inhibiting endothelial inflammation via ErbB4/Akt/NF-κB pathway (Figure 4).79 These findings strongly suggest that Nrg4 can protect against atherosclerosis, at least, partly by its anti-inflammatory effect.
Conclusions
In conclusion, as a novel adipocytokine, Nrg4 has been revealed to be closely related to T2DM-related vascular complications by regulating insulin sensitivity, inflammation, and glucose and lipid metabolism, and may be used as a good biomarker for the early detection of diabetic vascular complications. However, information about the relationship between Nrg4 and diabetic vascular complications is little, and further clinical and experimental studies are urgently need to determine whether Nrg4 can be used as a novel biomarker and therapeutic agent for type 2 diabetic vascular complications, thus to make it possible to develop therapeutic drugs targeting Nrg4-ErbB4 pathway. Therefore, Nrg4 may be a potential endocrine therapeutic target for type 2 diabetic vascular complications in the future. We hope this review can be helpful to researchers interested in the association between Nrg4 level and diabetic vascular complications.
Abbreviations
Nrg4, Neuregulin 4; BAT, brown adipose tissue; T2DM, type 2 diabetes mellitus; CAD, coronary artery disease; PAD, peripheral arterial disease; EGF, epidermal growth factor; WAT, white adipose tissue; PI3K/Akt, phosphatidylinositol-3 kinase/protein kinase B; ErbB4, erb-b2 receptor tyrosine kinase 4; MAPK, mitogen-activated protein kinases; STAT5, signal transducer and activator of transcription 5; NAFLD, nonalcoholic fatty liver disease; CVD, cardiovascular disease; CIMT, carotid intima-media thickness; ACS, acute coronary syndrome; SHR, spontaneous hypertension rats; CHD, coronary heart disease; ABI, ankle-brachial index; mTOR, mechanistic target of rapamycin; DN, diabetic nephropathy; DKD, diabetic kidney disease; AGEs, advanced glycosylation end products; TIF, tubulointerstitial fibrosis; ECM, extracellular matrix; TNF-R1, tumor necrosis factor-receptor 1; DPN, diabetic peripheral neuropathy; VPT, vibratory perception threshold; DR, diabetic retinopathy; PKC, protein kinase C; VEGF, vascular endothelial growth factor; NF-kB, nuclear transcription factor-kappa B; IR, insulin resistance; NO, nitric oxide; HFD, high-fat diet; GLUT4, glucose transporter four; GSV, GLUT4 storage vesicle; hs-CRP, high-sensitivity C-reactive protein; TNF-α, tumor necrosis factor alpha; MCP-1, monocyte chemotactic protein 1; 8-iso-PGF2α, 8-iso-prostaglandin F2α; GGT, gamma-glutamyl transferase.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was funded by grants from the key Research and Development Program of Science and Technology Department of Sichuan Province (2022YFS0612).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Saeedi P, Petersohn I, Salpea P, et al. IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabet Res Clin Pract. 2019;157:107843. doi:10.1016/j.diabres.2019.107843
2. Rosell M, Kaforou M, Frontini A, et al. Brown and white adipose tissues: intrinsic differences in gene expression and response to cold exposure in mice. Am J Physiol Endocrinol Metab. 2014;306(8):E945–64. doi:10.1152/ajpendo.00473.2013
3. Wang GX, Zhao XY, Meng ZX, et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med. 2014;20(12):1436–1443. doi:10.1038/nm.3713
4. Cai C, Lin M, Xu Y, Li X, Yang S, Zhang H. Association of circulating neuregulin 4 with metabolic syndrome in obese adults: a cross-sectional study. BMC Med. 2016;14(1):165. doi:10.1186/s12916-016-0703-6
5. Kang YE, Kim JM, Choung S, et al. Comparison of serum Neuregulin 4 (Nrg4) levels in adults with newly diagnosed type 2 diabetes mellitus and controls without diabetes. Diabet Res Clin Pract. 2016;117:1–3. doi:10.1016/j.diabres.2016.04.007
6. Chen L, Xuan Y, Zhu Y, et al. Adipocyte secreted NRG4 ameliorates age-associated metabolic dysfunction. Biochem Pharmacol. 2024;225:116327. doi:10.1016/j.bcp.2024.116327
7. Schumacher MA, Dennis IC, Liu CY, et al. NRG4-ErbB4 signaling represses proinflammatory macrophage activity. Am J Physiol Gastrointest Liver Physiol. 2021;320(6):G990–G1001. doi:10.1152/ajpgi.00296.2020
8. Chen M, Zhu J, Luo H, Mu W, Guo L. The journey towards physiology and pathology: tracing the path of neuregulin 4. Genes Dis. 2023;11(2):687–700. doi:10.1016/j.gendis.2023.03.021
9. Ma Y, Gao M, Liu D. Preventing high fat diet-induced obesity and improving insulin sensitivity through Neuregulin 4 gene transfer. Sci Rep. 2016;6:26242. doi:10.1038/srep26242
10. Pfeifer A. NRG4: an endocrine link between brown adipose tissue and liver. Cell Metab. 2015;21(1):13–14. doi:10.1016/j.cmet.2014.12.008
11. Bublil EM, Yarden Y. The EGF receptor family: spearheading a merger of signaling and therapeutics. Curr Opin Cell Biol. 2007;19(2):124–134. doi:10.1016/j.ceb.2007.02.008
12. Wang GX, Zhao XY, Lin JD. The brown fat secretome: metabolic functions beyond thermogenesis. Trends Endocrinol Metab. 2015;26(5):231–237. doi:10.1016/j.tem.2015.03.002
13. Guo L, Zhang P, Chen Z, et al. Hepatic neuregulin 4 signaling defines an endocrine checkpoint for steatosis-to-NASH progression. J Clin Invest. 2017;127(12):4449–4461. doi:10.1172/JCI96324
14. Zhong M, Tian X, Sun Q, et al. Correlation of asprosin and Nrg-4 with type 2 diabetes mellitus complicated with coronary heart disease and the diagnostic value. BMC Endocr Disord. 2023;23(1):61. doi:10.1186/s12902-023-01311-8
15. Yan P, Zhang Z, Miao Y, Xu Y, Zhu J, Wan Q. Changes of circulating neuregulin 4 and its relationship with 25-hydroxy vitamin D and other diabetic vascular complications in patients with diabetic peripheral neuropathy. Diabetol Metab Syndr. 2020;12:42. doi:10.1186/s13098-020-00550-2
16. Yan P, Xu Y, Zhang Z, et al. Decreased plasma neuregulin 4 levels are associated with peripheral neuropathy in Chinese patients with newly diagnosed type 2 diabetes: a cross-sectional study. Cytokine. 2019;113:356–364. doi:10.1016/j.cyto.2018.10.007
17. Kocak MZ, Aktas G, Atak BM, et al. Is Neuregulin-4 a predictive marker of microvascular complications in type 2 diabetes mellitus? Eur J Clin Invest. 2020;50(3):e13206. doi:10.1111/eci.13206
18. Ding S, Yang Y, Zheng Y, et al. Diagnostic value of the combined measurement of serum HCY and NRG4 in type 2 diabetes mellitus with early complicating diabetic nephropathy. J Pers Med. 2023;13(3):556. doi:10.3390/jpm13030556
19. Jiang J, Lin M, Xu Y, et al. Circulating neuregulin 4 levels are inversely associated with subclinical cardiovascular disease in obese adults. Sci Rep. 2016;6:36710. doi:10.1038/srep36710
20. Nakanishi K, Daimon M, Yoshida Y, et al. Carotid intima-media thickness and subclinical left heart dysfunction in the general population. Atherosclerosis. 2020;305:42–49. doi:10.1016/j.atherosclerosis.2020.05.019
21. Lorenz MW, Polak JF, Kavousi M, et al. Carotid intima-media thickness progression to predict cardiovascular events in the general population (the PROG-IMT collaborative project): a meta-analysis of individual participant data. Lancet. 2012;379(9831):2053–2062. doi:10.1016/S0140-6736(12)60441-3
22. Tian QP, Liu ML, Tang CS, Xue L, Pang YZ, Qi YF. Association of circulating Neuregulin-4 with presence and severity of coronary artery disease. Int Heart J. 2019;60(1):45–49. doi:10.1536/ihj.18-130
23. Rahimzadeh M, Farshidi N, Naderi N, Farshidi H, Montazerghaem H. Clinical significance of serum concentrations of neuregulin-4, in acute coronary syndrome. Sci Rep. 2020;10(1):5797. doi:10.1038/s41598-020-62680-x
24. Liu SQ, Tefft BJ, Roberts DT, et al. Cardioprotective proteins upregulated in the liver in response to experimental myocardial ischemia. Am J Physiol Heart Circ Physiol. 2012;303(12):H1446–58. doi:10.1152/ajpheart.00362.2012
25. Yang Z, Yaling W, Tao L, Mingyue R, Yongjun L. Cardioprotective effect of NRG-4 gene expression on spontaneous hypertension rats and its mechanism through mediating the activation of ErbB signaling pathway. Cell Mol Biol. 2022;68(1):89–101. doi:10.14715/cmb/2022.68.1.12
26. Barnes JA, Eid MA, Creager MA, Goodney PP. Epidemiology and risk of amputation in patients with diabetes mellitus and peripheral artery disease. Arterioscler Thromb Vasc Biol. 2020;40(8):1808–1817. doi:10.1161/ATVBAHA.120.314595
27. Lau JF, Weinberg MD, Olin JW. Peripheral artery disease. Part 1: clinical evaluation and noninvasive diagnosis. Nat Rev Cardiol. 2011;8(7):405–418. doi:10.1038/nrcardio.2011.66
28. Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006;47(5):921–929. doi:10.1016/j.jacc.2005.09.065
29. Yokoyama H, Sone H, Honjo J, et al. Relationship between a low ankle brachial index and all-cause death and cardiovascular events in subjects with and without diabetes. J Atheroscler Thromb. 2014;21(6):574–581.
30. Lange S, Diehm C, Darius H, et al. High prevalence of peripheral arterial disease and low treatment rates in elderly primary care patients with diabetes. Exp Clin Endocrinol Diabetes. 2004;112(10):566–573. doi:10.1055/s-2004-830408
31. Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi:10.1016/S0140-6736(10)60484-9
32. Prandi FR, Evangelista I, Sergi D, Palazzuoli A, Romeo F. Mechanisms of cardiac dysfunction in diabetic cardiomyopathy: molecular abnormalities and phenotypical variants. Heart Fail Rev. 2023;28(3):597–606. doi:10.1007/s10741-021-10200-y
33. Fernandez CJ, Shetty S, Pappachan JM. Diabetic cardiomyopathy: emerging therapeutic options. World J Diabetes. 2024;15(8):1677–1682. doi:10.4239/wjd.v15.i8.1677
34. Zech ATL, Singh SR, Schlossarek S, Carrier L. Autophagy in cardiomyopathies. Biochim Biophys Acta Mol Cell Res. 2020;1867(3):118432. doi:10.1016/j.bbamcr.2019.01.013
35. Wang H, Wang L, Hu F, et al. Neuregulin-4 attenuates diabetic cardiomyopathy by regulating autophagy via the AMPK/mTOR signalling pathway. Cardiovasc Diabetol. 2022;21(1):205. doi:10.1186/s12933-022-01643-0
36. Geissler A, Ryzhov S, Sawyer DB. Neuregulins: protective and reparative growth factors in multiple forms of cardiovascular disease. Clin Sci. 2020;134(19):2623–2643. doi:10.1042/CS20200230
37. Bhattacharjee N, Barma S, Konwar N, Dewanjee S, Manna P. Mechanistic insight of diabetic nephropathy and its pharmacotherapeutic targets: an update. Eur J Pharmacol. 2016;791:8–24. doi:10.1016/j.ejphar.2016.08.022
38. Sharaf El Din UAA, Salem MM, Abdulazim DO. Diabetic nephropathy: time to withhold development and progression - A review. J Adv Res. 2017;8(4):363–373. doi:10.1016/j.jare.2017.04.004
39. Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci. 2013;124(3):139–152. doi:10.1042/CS20120198
40. Li H, Peng X, Wang Y, et al. Atg5-mediated autophagy deficiency in proximal tubules promotes cell cycle G2/M arrest and renal fibrosis. Autophagy. 2016;12(9):1472–1486. doi:10.1080/15548627.2016.1190071
41. Shi J, Xu W, Zheng R, Miao H, Hu Q. Neuregulin 4 attenuate tubulointerstitial fibrosis and advanced glycosylation end products accumulation in diabetic nephropathy rats via regulating TNF-R1 signaling. Am J Transl Res. 2019;11(9):5501–5513.
42. Kralisch S, Hoffmann A, Klöting N, et al. The brown fat-secreted adipokine neuregulin 4 is decreased in human and murine chronic kidney disease. Eur J Endocrinol. 2019;181(2):151–159. doi:10.1530/EJE-19-0017
43. Deng J, Yang Q, Zhu W, et al. Neuregulin 4 attenuates podocyte injury and proteinuria in part by activating AMPK/mTOR-mediated autophagy in mice. J Cell Biochem. 2024;125(10):e30634. doi:10.1002/jcb.30634
44. Selvarajah D, Kar D, Khunti K, et al. Diabetic peripheral neuropathy: advances in diagnosis and strategies for screening and early intervention. Lancet Diabetes Endocrinol. 2019;7(12):938–948. doi:10.1016/S2213-8587(19)30081-6
45. Sun J, Wang Y, Zhang X, Zhu S, He H. Prevalence of peripheral neuropathy in patients with diabetes: a systematic review and meta-analysis. Prim Care Diabetes. 2020;14(5):435–444. doi:10.1016/j.pcd.2019.12.005
46. Oh TJ, Lee JE, Choi SH, Jang HC. Association between body fat and diabetic peripheral neuropathy in middle-aged adults with type 2 diabetes mellitus: a preliminary report. J Obes Metab Syndr. 2019;28(2):112–117. doi:10.7570/jomes.2019.28.2.112
47. Feng Y, Chen L, Luo Q, Wu M, Chen Y, Shi X. Involvement of microRNA-146a in diabetic peripheral neuropathy through the regulation of inflammation. Drug Des Devel Ther. 2018;12:171–177. doi:10.2147/DDDT.S157109
48. Doupis J, Lyons TE, Wu S, Gnardellis C, Dinh T, Veves A. Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. J Clin Endocrinol Metab. 2009;94(6):2157–2163. doi:10.1210/jc.2008-2385
49. Baum P, Toyka KV, Blüher M, Kosacka J, Nowicki M. Inflammatory mechanisms in the pathophysiology of diabetic peripheral neuropathy (DN)-new aspects. Int J Mol Sci. 2021;22(19):10835. doi:10.3390/ijms221910835
50. Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127–137. doi:10.1038/35052073
51. Paramo B, Wyatt S, Davies AM. An essential role for neuregulin-4 in the growth and elaboration of developing neocortical pyramidal dendrites. Exp Neurol. 2018;302:85–92. doi:10.1016/j.expneurol.2018.01.002
52. Liu Y, Ford BD, Mann MA, Fischbach GD. Neuregulin-1 increases the proliferation of neuronal progenitors from embryonic neural stem cells. Dev Biol. 2005;283(2):437–445. doi:10.1016/j.ydbio.2005.04.038
53. Hayes NV, Newsam RJ, Baines AJ, Gullick WJ. Characterization of the cell membrane-associated products of the Neuregulin 4 gene. Oncogene. 2008;27(5):715–720. doi:10.1038/sj.onc.1210689
54. Lee R, Wong TY, Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015;2:17. doi:10.1186/s40662-015-0026-2
55. Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi:10.1016/S0140-6736(09)62124-3
56. Montesano G, Ometto G, Higgins BE, et al. Evidence for structural and functional damage of the inner retina in diabetes with no diabetic retinopathy. Invest Ophthalmol Vis Sci. 2021;62(3):35. doi:10.1167/iovs.62.3.35
57. Zeng Y, Cao D, Yu H, et al. Early retinal neurovascular impairment in patients with diabetes without clinically detectable retinopathy. Br J Ophthalmol. 2019;103(12):1747–1752. doi:10.1136/bjophthalmol-2018-313582
58. Sohn EH, van Dijk HW, Jiao C, et al. Retinal neurodegeneration may precede microvascular changes characteristic of diabetic retinopathy in diabetes mellitus. Proc Natl Acad Sci U S A. 2016;113(19):E2655–64. doi:10.1073/pnas.1522014113
59. Kaur R, Kaur M, Singh J. Endothelial dysfunction and platelet hyperactivity in type 2 diabetes mellitus: molecular insights and therapeutic strategies. Cardiovasc Diabetol. 2018;17(1):121. doi:10.1186/s12933-018-0763-3
60. Dhananjayan R, Koundinya KS, Malati T, Kutala VK. Endothelial dysfunction in type 2 diabetes mellitus. Indian J Clin Biochem. 2016;31(4):372–379. doi:10.1007/s12291-015-0516-y
61. Ruan H, Miles PD, Ladd CM, et al. Profiling gene transcription in vivo reveals adipose tissue as an immediate target of tumor necrosis factor-alpha: implications for insulin resistance. Diabetes. 2002;51(11):3176–3188. doi:10.2337/diabetes.51.11.3176
62. Muniyappa R, Quon MJ. Insulin action and insulin resistance in vascular endothelium. Curr Opin Clin Nutr Metab Care. 2007;10(4):523–530. doi:10.1097/MCO.0b013e32819f8ecd
63. Chen Z, Wang GX, Ma SL, et al. Nrg4 promotes fuel oxidation and a healthy adipokine profile to ameliorate diet-induced metabolic disorders. Mol Metab. 2017;6(8):863–872. doi:10.1016/j.molmet.2017.03.016
64. Díaz-Sáez F, Blanco-Sinfreu C, Archilla-Ortega A, et al. Neuregulin 4 downregulation induces insulin resistance in 3T3-L1 adipocytes through inflammation and autophagic degradation of GLUT4 vesicles. Int J Mol Sci. 2021;22(23):12960. doi:10.3390/ijms222312960
65. South JC, Blackburn E, Brown IR, Gullick WJ. The neuregulin system of ligands and their receptors in rat islets of langerhans. Endocrinology. 2013;154(7):2385–2392. doi:10.1210/en.2012-2133
66. Martínez C, Latorre J, Ortega F, et al. Serum neuregulin 4 is negatively correlated with insulin sensitivity in humans and impairs mitochondrial respiration in HepG2 cells. Front Physiol. 2022;13:950791. doi:10.3389/fphys.2022.950791
67. Comas F, Martínez C, Sabater M, et al. Neuregulin 4 is a novel marker of beige adipocyte precursor cells in human adipose tissue. Front Physiol. 2019;10:39. doi:10.3389/fphys.2019.00039
68. Gasmi A, Noor S, Menzel A, Doşa A, Pivina L, Bjørklund G. Obesity and insulin resistance: associations with chronic inflammation, genetic and epigenetic factors. Curr Med Chem. 2021;28(4):800–826. doi:10.2174/0929867327666200824112056
69. Yan PJ, Xu Y, Wan Q, et al. Decreased plasma neuregulin 4 concentration is associated with increased high-sensitivity C-reactive protein in newly diagnosed type 2 diabetes mellitus patients: a cross-sectional study. Acta Diabetol. 2017;54(12):1091–1099. doi:10.1007/s00592-017-1044-4
70. Farshidi H, Naderi N, Montazerghaem H, Farshidi N, Rahimzadeh M. Negative correlation between Neuregulin-4 and IL-9 serum levels in patients with coronary artery disease. Endocr Metab Immune Disord Drug Targets. 2021;21(11):2068–2074. doi:10.2174/1871530321666210119115527
71. Schumacher MA, Hedl M, Abraham C, et al. ErbB4 signaling stimulates pro-inflammatory macrophage apoptosis and limits colonic inflammation. Cell Death Dis. 2017;8(2):e2622. doi:10.1038/cddis.2017.42
72. Nugroho DB, Ikeda K, Kajimoto K, Hirata KI, Emoto N. Activation of neuregulin-4 in adipocytes improves metabolic health by enhancing adipose tissue angiogenesis. Biochem Biophys Res Commun. 2018;504(2):427–433. doi:10.1016/j.bbrc.2018.08.197
73. Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20(7):1126–1167. doi:10.1089/ars.2012.5149
74. Son SM. Reactive oxygen and nitrogen species in pathogenesis of vascular complications of diabetes. Diabetes Metab J. 2012;36(3):190–198. doi:10.4093/dmj.2012.36.3.190
75. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi:10.1038/414813a
76. Slattery ML, Pellatt DF, Mullany LE, Wolff RK. Differential gene expression in colon tissue associated with diet, lifestyle, and related oxidative stress. PLoS One. 2015;10(7):e0134406. doi:10.1371/journal.pone.0134406
77. Roth Flach RJ, Skoura A, Matevossian A, et al. Endothelial protein kinase MAP4K4 promotes vascular inflammation and atherosclerosis. Nat Commun. 2015;6:8995. doi:10.1038/ncomms9995
78. Meng B, Li Y, Ding Y, et al. Myeloid-derived growth factor inhibits inflammation and alleviates endothelial injury and atherosclerosis in mice. Sci Adv. 2021;7(21):eabe6903. doi:10.1126/sciadv.abe6903
79. Shi L, Li Y, Xu X, et al. Brown adipose tissue-derived Nrg4 alleviates endothelial inflammation and atherosclerosis in male mice. Nat Metab. 2022;4(11):1573–1590. doi:10.1038/s42255-022-00671-0
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.