Back to Journals » OncoTargets and Therapy » Volume 18
The Potential Role and Effective Components of Sanyeqing as the Potential Therapeutic Candidates for IBD and CRC
Authors Chen C, Chen L, Zheng W, Dai Y
Received 27 January 2025
Accepted for publication 1 May 2025
Published 7 July 2025 Volume 2025:18 Pages 779—788
DOI https://doi.org/10.2147/OTT.S516341
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Lukas Hawinkels
Chaoying Chen, Lin Chen, Weifeng Zheng, Yiyang Dai
Department of Gastroenterology, The Fourth Affiliated Hospital ZheJiang University School of Medicine, Yiwu, Zhejiang, 322000, People’s Republic of China
Correspondence: Chaoying Chen, Email [email protected]
Abstract: Inflammatory bowel disease (IBD) is a family of chronic inflammatory diseases such as Crohn’s disease (CD) and ulcerative colitis (UC). Among the serious malignancies that can arise from IBD, colorectal cancer is particularly prevalent. Individuals suffering from both IBD and CRC often endure similar symptoms, which include diarrhea, rectal bleeding, abdominal discomfort, weight decline, and profound exhaustion. Sanyeqing is a traditional herbaceous medicinal plant with anti-tumor, anti-inflammatory, analgesic, heat-clearing, detoxifying, and liver-protecting effects. Here, we summarize the possible molecular mechanisms of IBD and CRC, and summarize the potential role of Sanyeqing in clinical therapy for IBD and CRC. Investigating the etiology of enteritis and intestinal cancer, as well as exploring Sanyeqing’s potential as a preventive and therapeutic agent, is of paramount importance in the battle against these diseases.
Keywords: inflammatory bowel disease, colorectal cancer, sanyeqing, anti-inflammatory, anti-tumor
Introduction
Inflammatory Bowel Disease (IBD) is an extremely common disease with approximately 3–5 billion cases per year worldwide.1 IBD is a complex condition influenced by various factors, including genetic predispositions, immune responses, environmental stimuli, and the composition of the gut microbiome. It represents a form of gastrointestinal impairment resulting from persistent inflammation. IBD mainly includes CD and UC.2 From a clinical perspective, individuals diagnosed with either CD or UC commonly present with symptoms that include diarrhea, rectal hemorrhage, and abdominal discomfort. However, notable distinctions exist in terms of the anatomical sites affected and the severity of inflammation, in addition to the complications and incidence rates associated with these two disorders. Moreover, patients with IBD also usually display signs including loss of weight and severe exhaustion. Some patients with severe symptoms must undergo surgery to repair or resect damaged intestines.3 Moreover, patients with persistent inflammation exhibit a heightened likelihood of developing colorectal cancer in contrast to individuals without colitis.4
Sanyeqing (Tetrastigma hemsleyanum), as a traditional Chinese medicinal herb, has recently garnered significant attention for its therapeutic potential in managing various diseases.5,6 Studies have demonstrated that Sanyeqing exhibits anti-inflammatory, antioxidant, and immunomodulatory properties, which confer promising applications in the treatment of IBD and colorectal cancer (CRC).7,8 Notably, in IBD patients, it may alleviate clinical symptoms by suppressing inflammatory responses and promoting mucosal repair.9,10 Furthermore, research has revealed that Sanyeqing inhibits proliferation and induces apoptosis in CRC cells, suggesting its potential in CRC prevention and treatment.11 Although preliminary studies have shown beneficial effects of Sanyeqing on both IBD and CRC, the precise underlying mechanisms and clinical applications require further investigation through well-designed preclinical and clinical trials. Here, we review the pathogenesis of IBD and CRC as well as the potential function of Sanyeqing as a therapeutic agent for IBD and CRC.
However, the exact molecular pathways involved in IBD remain unknown. The etiology of IBD is generally assumed to be multifactorial and multistep.12 For example, environmental factors trigger abnormal immune responses with inflammatory reactions in susceptible individuals and alterations in the intestinal microbiota can induce IBD.13 In addition, the inner intestinal epithelium not only absorbs nutrients but also undertakes the function of preventing harmful microorganisms from invading and interacting with commensal bacteria.14 The population and abundance of intestinal microbiota are regulated by the intestinal immune system to maintain homeostasis in the intestinal tract. For various reasons, disruption of this homeostasis naturally leads to intestinal diseases, such as enteritis and intestinal cancer.15 The exact cause of IBD remains unclear. Researchers postulate that alterations in specific environmental or intrinsic factors can lead to a compromise in the host’s immune response, damage to the intestinal barrier, and disruption in the equilibrium of gut microbiota, ultimately resulting in the onset of chronic inflammation.16
The Pathogenesis of IBD and Intestinal Cancer
The connection between the integrity of intestinal epithelial cells and the occurrence of enteritis is significant. Intestinal epithelial cells (IECs) consist of a variety of cell types, which include enterocytes, goblet cells, enteroendocrine cells, Paneth cells, M cells, and intestinal epithelial stem cells.17 These cells utilize tight junctions to establish a protective barrier separating the intestinal lumen from the underlying intestinal tissue. In conjunction with the mucus layer, which is enriched with antimicrobial peptides secreted by the intestinal epithelium, they contribute to preventing the intrusion of microorganisms from the intestinal lumen.18 The functionality of the intestinal epithelial barrier is primarily upheld by the tight junctions that connect intestinal epithelial cells. When these tight junctions are compromised, there is an elevation in the permeability of the intestinal epithelium. This disruption can lead to significant exposure of intestinal contents or microbial infiltration, which triggers and sustains an inflammatory response, ultimately resulting in enteritis. For example, in mice lacking the N-cadherin gene or NOD1 and NOD2, the barrier function of the intestinal epithelium is disrupted, and mice develop symptoms resembling intestinal inflammation.19 In addition to using tight junctions to form a barrier to isolate intestinal epithelial cells from external bacterial invasion, some intestinal epithelial cells can block the invasion of microorganisms by secreting antimicrobial peptides and mucus to form a mucus layer. The mucosal layer is primarily constituted of glycosylated mucins produced by goblet cells, along with defensins released by Paneth and intestinal epithelial cells. Mice that are deficient in the Muc2 gene exhibit a tendency to develop spontaneous enteritis as they mature. Furthermore, a reduction in the quantity of goblet cells, along with a thinner mucus layer, has been noted in individuals diagnosed with ulcerative colitis.20
The Connection Between Gut Microbiota and Enteritis
Typically, the gut bacteria encompass various categories, including gram-negative bacteria such as Bacteroidetes, gram-positive bacteria like Firmicutes, in addition to other related subclades, including Proteobacteria, Actinomycetes, Clostridium, and Verrucobacteria.21,22 Gut microbes play an important role in digestion, nutrient metabolism, self-development, immunity, and disease generation. In recent years, scientists worldwide have turned their attention to the study of gut microbes, and numerous investigations have demonstrated a direct correlation between gut microbiota and the development of a range of diseases, such as cardiovascular conditions, obesity, neurological disorders, and atherosclerosis.23–26 In recent times, advancements in genetic and metagenomic methodologies have facilitated a deeper understanding of the connections between intestinal microbiota and IBDs, and inflammation-related intestinal cancer has received extensive attention and has become an emerging therapeutic target for tumor treatment.10,27–29
Compared to healthy people, the mucosa-associated microbiota of IBD patients is greatly reduced in diversity and quantity, and dysbiosis of the microbial population structure may also trigger intestinal inflammation in some cases.30 Research has indicated that commensal bacteria significantly contribute to the onset and progression of enteritis: first, in some patient populations, the symptoms of enteritis can be relieved by inhibiting intestinal microbiota through antibiotic treatment;31 second, the immune system and mutations in genes related to bacterial surveillance, such as NOD2, and genes related to T cells, such as IL23R, are highly correlated with the occurrence and development of enteritis, and the majority of animal models utilized for the study of colitis necessitate the collaborative action of commensal bacteria to induce inflammation in the intestine.32 Based on the imbalance in the composition and number of intestinal microbiota in patients with enteritis, researchers have used the treatment technology of fecal microbiota transplantation (FMT) to treat enteritis. Certain investigators have conducted an analysis on the efficacy of fecal microbiota transplantation (FMT) and determined that the success rate, defined as the alleviation of symptoms, for adult enteritis patients receiving FMT was 77.8%. Conversely, in pediatric patients with enteritis, the success rate for symptom relief reached an impressive 100%.
Compared to the feces of clinical colorectal cancer patients and healthy people, it was found that the intestinal microbiota composition of colorectal cancer patients changed significantly, and the compositional changes promote tumorigenesis.33,34 Inflammation is accompanied by IBD, gastrointestinal infections, and cancer.35 At this juncture, the structure of the intestinal microbial community underwent significant alterations, leading to a gradual reduction in the population of beneficial bacteria, which were subsequently supplanted by potentially harmful bacteria. Consequently, this dysbiosis of the intestinal microbiota is recognized as a key indicator of heightened intestinal inflammation. Fecal transplantation has also been used clinically to treat ulcerative colitis. Consequently, a deeper understanding of the molecular mechanisms that contribute to the dysregulation of intestinal microbiota is essential for the advancement of more efficient therapeutic approaches aimed at addressing IBD and inflammation-associated colorectal cancer.
The Correlation Between the Innate Immune Recognition Mechanism and Enteritis
The innate immune system serves as the first line of defense against external pathogens, playing a crucial role in human immunity. This system offers swift and nonspecific defense to the host through the recognition of pathogen-associated molecular patterns. Within the intestinal context, the innate immune system encompasses a variety of cell types, including intestinal epithelial cells, macrophages, monocytes, neutrophils, eosinophils, basophils, dendritic cells (DC), and natural killer cells (NK).
Intestinal inflammation has a significant association with the emergence and progression of tumors. CRC, recognized as a malignant neoplasm characterized by elevated incidence and mortality rates, ranks among the most prevalent cancers affecting the gastrointestinal tract globally.36,37 The latest global cancer epidemiological data show that the morbidity and mortality of colorectal cancer rank fourth and second among malignant tumors, respectively, and the prevalence of this cancer shows an increasing trend year by year.38 There were over 1.9 million new cases and resulted in approximately 935,000 fatalities globally.39 Individuals diagnosed with IBDs, including ulcerative colitis and Crohn’s disease, exhibit a notably heightened risk for the development of colorectal cancer.40,41 According to various clinical follow-up investigations, the administration of nonsteroidal anti-inflammatory agents, such as low-dose aspirin, has been shown to substantially diminish the occurrence of colon cancer.42 When aspirin was administered to patients with colon tumors or a history of colon tumors, the growth rate of the tumors slowed, and the recurrence rate after surgical resection was reduced.43 Celecoxib is a non-steroidal anti-inflammatory drug (NSAID) that specifically inhibits the cyclooxygenase-2 (COX-2) enzyme, also performed well in suppresses the recurrence rate of colorectal cancer patients. Colitis-carcinoma transformation is a complex pathological process that involves multiple genes, stages, and steps. Genome-wide association study (GWAS) results shown that the inactivation of tumor suppressor genes, which play key regulatory roles in tumor progression, results in significant spatiotemporal differences in the pathogenesis of sporadic colon cancer and inflammation-related colorectal cancer.44 Although some progress has been made, the specific molecular mechanisms and effective therapeutic drugs for inflammation-associated colon cancer remain to be elucidated.
In summary, a compromised innate immune response can result in an inability to effectively suppress microbial activity and may convey erroneous signals to the adaptive immune system during antigen presentation and the secretion of inflammatory mediators. This dysfunction can consequently facilitate the onset and progression of enteritis. Therefore, researchers regard the regulation of innate immune activity and homeostasis as important for the treatment of IBD.
The Molecular Mechanism and Potential Function of Sanyeqing in IBD and CRC
Tetrastigma hemsleyanum Diels et Gilg, commonly referred to as Sanyeqing (SYQ), is a perennial herbaceous climbing vine belonging to the family Vitis, which is a rare medicinal plant unique in our country (Figure 1). SYQ is found in the sparse forests of valleys or on stone walls in the shade and is distributed from the Yangtze River Basin to the southern provinces of China.45 The SYQ in Fujian, Hunan, Yunnan, Guangdong and Guangxi province is ivy SYQ, and the ones in Zhejiang and Jiangxi is Wisteria SYQ. SYQ is slightly bitter and flat. It is used as a medicine, along with tubers or whole plants. It has the reputation of “King of Medicine”, “Anti-cancer Herb” and “Plant Antibiotic”. It is used to treat febrile convulsions, whooping cough, pneumonia, and asthma in children. It has anti-cancer, anti-tumor, anti-inflammatory, analgesic, heat-clearing, detoxifying, and liver-protecting effects, and has few toxic and side effects.45
 |
Figure 1 Sanyeqing is shown. The aboveground part and the blocky root. |
The effective material basis of Chinese herbal medicine is a natural product, which has the characteristics of rich structural types, unique pharmacodynamic activity, synergistic effects of ingredients, and diverse action targets, and is an important source of innovative drugs.46 Different researchers have used different methods and solvents to conduct experiments on the roots and aerial parts of SYQ and studied their chemical components.47 The types and quantities of the compounds obtained were different, indicating that there is no unified standard for the extraction and separation of chemical components in SYQ. This article reviews the mechanism and targets of SYQ extract in the treatment of enteritis and intestinal cancer, and these preclinical findings provide mechanistic insights for further clinical exploration of Tetrastigma hemsleyanum-derived components in IBD and CRC management.
The Role of Tetrastigma Hemsleyanum Diels & Gilg in Managing IBD and Colorectal Cancer
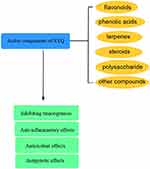
Tetrastigma hemsleyanum Diels et Gilg is a perennial climbing vine extensively utilized in traditional Chinese medicine for managing febrile disorders, hepatitis, and inflammatory conditions.45 The plant’s medicinal properties stem from its diverse phytochemical constituents, particularly flavonoids, phenolic acids, polysaccharides, triterpenoids, and steroids, as identified through phytochemical analyses. These compounds work together to produce a variety of pharmacological benefits such as tumor suppression, inflammation reduction, antioxidant effects, and immune system regulation.48 Proanthocyanidin, a type of polyphenolic compound, is particularly abundant in the purple leaves compared to the green leaves of T. hemsleyanum49 and researchers have identified forty-two different chemical constituents in this species, ranging from flavonoids and phenolic acids to polysaccharides, organic acids, fatty acids, terpenoids, steroids, and amino acids. Flavonoids and polysaccharides are highlighted as the primary active ingredients in T. hemsleyanum and have been extensively studied.50 Certain compounds, including chlorogenic acid, quinic acid, catechin, kaempferol 3-rutinoside, apigenin-8-C-glucoside, and linolenic acid, have been associated with anticancer properties, while chlorogenic acid, quercetin, quinic acid, kaempferol 3-rutinoside, rutin, apigenin-8-C-glucoside, and linolenic acid are known for their ability to combat inflammation51 (Figure 2).
Polysaccharides are a complex mix of sugars, predominantly glucose, but also include mannose, glucuronic acid, rhamnose, galacturonic acid, galactose, and arabinose.52 Studies suggest that the polysaccharides extracted from Tetrastigma hemsleyanum have the capacity to upregulate the expression of proteins integral to intestinal tight junctions, which in turn reduces the permeability of the intestinal mucosa and improves the pathological state of intestinal epithelial cells. Moreover, SYQ-PA has been demonstrated to boost the count of beneficial probiotics, such as Lactobacillus, while reducing the abundance of harmful bacteria like Enterococcus. This bacterial balance contributes to the repair of the intestinal biological barrier, the rejuvenation of the intestinal mucosal barrier’s function, and the preservation of intestinal equilibrium.53 Additionally, the administration of SYQ has been seen to alleviate LPS-induced harm to the intestines and to decrease the secretion of inflammatory cytokines, including tumor necrosis factor-alpha (TNF-alpha), interleukin 1beta (IL-1beta), and interleukin 6 (IL-6). SYQ exerts a shielding effect against LPS-triggered intestinal mucosal barrier damage in septic mice by dampening the inflammatory reaction, intensifying the expression of tight junction proteins, inhibiting apoptosis in intestinal cells, and reshaping the composition of the intestinal microbiota.54 Furthermore, the polysaccharides extracted from SYQ (SYQP) markedly reduced the release of pro-inflammatory cytokines such as IL-6, TNF-α, and IFN-γ, and concurrently suppressed the excessive expression of TLR2 and TLR4, as well as the phosphorylation of NF-κB p65. SYQP elicits a dual immune response and alleviates LPS-induced acute respiratory distress syndrome in mice through the TLR2/TLR4-NF-kappaB, NLRP3/caspase, and JAK/STAT signaling pathways, underpinning the potential therapeutic application of SYQP.55 Additionally, SYQP reduces LPS-induced acute lung injury (ALI) by repressing inflammation and oxidative stress, achieved through the inhibition of the TLR4/COX-2/NF-κB signaling pathway.56 In a separate study, the isolated polysaccharide TTP-1 demonstrated efficacy in attenuating LPS-induced inflammation, cytotoxicity, genotoxicity, mitochondrial dysfunction, and oxidative stress in RAW264.7 cells. TTP-1’s anti-inflammatory effects were mediated through the regulation of COX-2, iNOS, and MAPKs pathways, and it also combated oxidative damage by activating the Nrf2-Keap1 and Sirt1-FoxO1 pathways in these cells.57 THP, another polysaccharide, exerted protective effects against H(2)O(2)-induced cytotoxicity in RAW264.7 cells by decreasing intracellular ROS, reducing the activity of catalase (CAT) and superoxide dismutase (SOD), while increasing lactate dehydrogenase (LDH) activity and raising malondialdehyde (MDA) levels. In vitro experiments have conclusively highlighted the antioxidative characteristics of polysaccharides from T. hemsleyanum roots.58 Thus, polysaccharides from the root of SYQ can inhibit IBD by reducing inflammatory responses and oxidative stress.
SYQ Possessed a Potential to Inhibit the Progression of CRC
The polysaccharides from Tetrastigma are composed of a diverse array of constituents, such as galacturonic acid (GalA), glucose (Glc), mannose (Man), arabinose (Ara), galactose (Gal), and rhamnose (Rha). The intake of SYQP has demonstrated its ability to augment the phagocytic capacity of macrophages, stimulate natural killer (NK) cells, and boost the levels of complement proteins and immunoglobulins, including IgG, IgA, and IgM. Moreover, it has been noted to counteract the immunosuppressive effects induced by CTX in mouse models, achieved through the regulation of intestinal microbiota and enhancing immunomodulatory activities in vivo.27 Another investigation revealed that a 200 mg/kg oral dose of SYQP facilitated tumor regression and fortified immune reactions in mice. The molecular underpinnings of this effect involve the interaction of SYQ-PA with TLR4, which stimulates the antigen-specific proliferation of spleen lymphocytes and elevates serum IgG levels in ovalbumin-vaccinated C57BL/6 mice.59 This suggests that SYQP may function as a competitive inhibitor of lipopolysaccharide (LPS) binding to toll-like receptor 4 (TLR4). Furthermore, the oral delivery of a 200 mg/kg dose of SYQP led to a 39.9% decrease in the progression of H22 tumors in mice, concomitant with an upsurge in the secretion of cytokines, such as tumor necrosis factor α (TNF-α) and interferon γ (IFN-γ).60 Additionally, SYQP suppressed breast cancer progression by promoting macrophage polarization of the M1 phenotype. The underlying mechanism revealed that SYQ-PA inhibits PPAR-γ-and-catenin-related pathways in macrophages.61
Moreover, other active components of SYQ showed antitumor effects. For example, the petroleum ether fraction (PEF) derived from SYQ demonstrated a capacity to suppress the proliferation and promote apoptosis of HeLa cells in a manner that is both dose- and time-dependent. This effect occurs through the activation of both extrinsic and intrinsic apoptotic pathways, alongside an increase in oxidative stress within cervical carcinoma HeLa cells.62 Furthermore, the ethyl acetate extract obtained from Tetrastigma hemsleyanum (EET) also exhibited inhibitory effects on cell proliferation and facilitated apoptosis in HepG2 and SMMC-7721 cells. This mechanism was linked to the downregulation of Caspase-3 protein expression and the upregulation of Bax expression.63
Flavonoids are acknowledged as key bioactive constituents of SYQ.64 A total of 22 distinct flavonoids were identified, with the majority exhibiting higher concentrations in the leaves compared to the root tubers.65 Moreover, the hairy roots of T. hemsleyanum were found to synthesize a greater amount of flavonoids than the true roots, demonstrating enhanced antioxidant properties in comparison to the latter.46 The flavonoids represent the most significant components of T. hemsleyanum, and their levels are primarily influenced by exposure to ultraviolet (UV) radiation.66
The aggregate of flavonoids isolated from Tetrastigma hemsleyanum (THTF), including but not limited to kaempferol 3-rutinoside, rutin, isoquercitrin, L-epicatechin, quercetin, astragalin, kaempferol 3-sambubioside, and catechin, exhibits a pronounced affinity for the amino acid residues within the binding domains of phosphatidylinositol 3-kinase (PI3K) and mammalian target of rapamycin (mTOR) proteins. This molecular interaction results in a marked attenuation of colorectal carcinoma proliferation by selectively inhibiting the PI3K/AKT/mTOR signaling axis, underscoring their therapeutic potential as chemopreventive agents in the management of colorectal cancer.11 Consequently, the flavonoid-rich fraction of T. hemsleyanum (THTF) may be conceptualized as a putative pharmacological entity for the treatment of gastrointestinal pathologies. Administration of THTF at a dose of 120 mg/kg has demonstrated efficacy in mitigating the neoplastic growth associated with colorectal cancer (CRC) and in safeguarding the intestinal homeostasis, as evidenced by the modulation of the phospho-NF-κB (p-p65)/NF-κB (p65) ratio and zonula occludens-1 (ZO-1) protein expression in HCT116 xenograft models. Moreover, THTF facilitates the in vitro proliferation of Bifidobacterium pseudolongum, and the resulting cell-free culture supernatants have been observed to exert an additional inhibitory effect on the proliferative and clonogenic potential of HCT116 cells. THTF appears to impede CRC tumor progression by restoring the equilibrium of the gut microbiota, reestablishing the homeostasis of fecal metabolites, and maintaining the integrity of the intestinal barrier.67 Furthermore, isoquercitrin has been found to selectively inhibit hepatocyte growth factor/scatter factor (HGF/SF)-induced tyrosine phosphorylation of the c-Met proto-oncogene receptor and to abrogate HGF/SF-mediated epithelial-mesenchymal transition (EMT) in vitro, as well as to inhibit the invasive and metastatic potential of HGF/SF-transfected NBT-II cells in vivo.68 Additional studies have revealed that total flavonoids significantly diminish the serum concentrations of transforming growth factor beta (TGF-β), prostaglandin E2 (PGE2), and cyclooxygenase 2 (COX-2) in tumor-bearing murine models, which may contribute to the suppression of immunosuppressive regulatory T cells (Tregs).69
The transformation and biochemical processing of the combined phenolic and flavonoid compounds result in the generation of derivatives such as quercetin-3-rutinoside, quercetin-3-glucoside, kaempferol-3-rutinoside, and kaempferol-3-glucoside. Additionally, within the framework of an oxidative stress experiment on rats treated with the extract from the root of SYQ, a marked increment in overall antioxidant proficiency (T-AOC), the enzymatic activity of SOD, GSH-Px, and the concentration of GSH was observed. Simultaneously, there was a discernible decline in the concentrations of MDA detected within both the plasma and various tissues. These observations suggest that the root extract from SYQ harbors potent antioxidant attributes.70
Till now, most of the studies primarily focused on in vitro or in vivo experimental experiments of single components of SYQ (such as SYQP or flavonoids), lacking exploration of the synergistic effects of the whole herbal efficacy components. Moreover, most experiments have not standardized the extraction process, leading to significant differences in the concentration and effects of active components across different literature, which poses challenges for clinical translation. Therefore, the future research directions for the medicinal plant SYQ include the following aspects: 1. Establishing a standardized system for the extraction and quality control of active components; 2. Exploring the pharmacodynamic network of the synergistic effects of the whole herbal components; 3. Identifying the target proteins and regulated cancer or inflammation-related signaling pathways of the main active components of SYQ at the cellular and animal levels; 4. Conducting preclinical toxicological assessments and clinical trials on SYQ; 5. Additionally, utilizing organoid models or single-cell sequencing techniques to elucidate the regulation of SYQ on intestinal epithelial cell heterogeneity may provide new insights for precision therapy.
Conclusion
In conclusion, this review systematically elaborates on the multi-target regulatory mechanisms of Tetrastigma hemsleyanum in inflammatory bowel disease (IBD) and colorectal cancer (CRC). Furthermore, the active components of T. hemsleyanum can modulate the gut microbiota structure (eg, increasing Bifidobacterium abundance) and reshape the host-microbe interaction network through short-chain fatty acid metabolism, revealing its potential blockade of IBD-CRC malignant transformation. Future research should focus on: developing a metabolomics-based quality control system for active components, establishing organoid models to elucidate multi-component synergistic targets, and designing targeted delivery systems to enhance bioavailability. Through interdisciplinary technological integration, T. hemsleyanum holds promise for transformation from a traditional herbal remedy into an innovative therapeutic agent for sequential IBD-CRC treatment, offering novel strategies for the prevention and treatment of gastrointestinal diseases.
Data Sharing Statement
The data generated in the present study may be requested from the corresponding author.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was received.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Massironi S, Vigano C, Palermo A, et al. Inflammation and malnutrition in inflammatory bowel disease. Lancet Gastroenterol Hepatol. 2023;8(6):579–590. doi:10.1016/S2468-1253(23)00011-0
2. Bao M, Wang K, Li J, et al. ROS scavenging and inflammation-directed polydopamine nanoparticles regulate gut immunity and flora therapy in inflammatory bowel disease. Acta Biomater. 2023;161:250–264. doi:10.1016/j.actbio.2023.02.026
3. Jori C, Chaudhary AA, Rashid S, et al. Biomaterial-based strategies for immunomodulation in IBD: current and future scenarios. J Mater Chem B. 2023;11(25):5668–5692. doi:10.1039/D3TB00276D
4. Fan Z, Tang P, Li C, et al. Fusobacterium nucleatum and its associated systemic diseases: epidemiologic studies and possible mechanisms. J Oral Microbiol. 2023;15(1):2145729. doi:10.1080/20002297.2022.2145729
5. Shang Y, Zhao M, Chen S, et al. Tetrastigma hemsleyanum polysaccharide combined with doxorubicin promote ferroptosis and immune function in triple-negative breast cancer. Int J Biol Macromol. 2024;275(Pt 1):133424. doi:10.1016/j.ijbiomac.2024.133424
6. Zhang Z, Chen Y, Wang S, et al. Effect of particle size on physical properties, dissolution, in vitro antioxidant activity, and in vivo hepatoprotective properties of tetrastigma hemsleyanum Diels et gilg powders. Pharmaceutics. 2024;16(11):1352. doi:10.3390/pharmaceutics16111352
7. Zhao M, Chen S, Xu J, et al. Alleviation of sepsis-induced lung and liver injury by polysaccharides from Tetrastigma hemsleyanum Diels et Gilg via suppression of TLR4/NF-kappaB/COX-2 pathway and modulation of immune checkpoint molecules. Mol Immunol. 2025;179:52–64. doi:10.1016/j.molimm.2025.02.002
8. Ruan Y, Zhu X, Shen J, Chen H, Zhou G. Mechanism of nicotiflorin in San-Ye-Qing rhizome for anti-inflammatory effect in ulcerative colitis. Phytomedicine. 2024;129:155564. doi:10.1016/j.phymed.2024.155564
9. Lin Y, Lv Y, Mao Z, et al. Polysaccharides from Tetrastigma Hemsleyanum Diels et Gilg ameliorated inflammatory bowel disease by rebuilding the intestinal mucosal barrier and inhibiting inflammation through the SCFA-GPR41/43 signaling pathway. Int J Biol Macromol. 2023;250:126167. doi:10.1016/j.ijbiomac.2023.126167
10. Bao X, Tang Y, Lv Y, et al. Tetrastigma hemsleyanum polysaccharide ameliorated ulcerative colitis by remodeling intestinal mucosal barrier function via regulating the SOCS1/JAK2/STAT3 pathway. Int Immunopharmacol. 2024;137:112404.
11. Zhai Y, Sun J, Sun C, et al. Total flavonoids from the dried root of Tetrastigma hemsleyanum Diels et Gilg inhibit colorectal cancer growth through PI3K/AKT/mTOR signaling pathway. Phytother Res. 2022;36(11):4263–4277. doi:10.1002/ptr.7561
12. Matsukawa T, Izawa K, Isobe M, et al. Ceramide-CD300f binding suppresses experimental colitis by inhibiting ATP-mediated mast cell activation. Gut. 2016;65(5):777–787. doi:10.1136/gutjnl-2014-308900
13. JSY H, Mok BW, Campisi L, et al. TOP1 inhibition therapy protects against SARS-CoV-2-induced lethal inflammation. Cell. 2021;184(10):2618–32e17.
14. Li Z, Li Y, Sun Q, et al. Targeting the pulmonary microbiota to fight against respiratory diseases. Cells. 2022;11(5):916.
15. Yang Q, Wang Y, Jia A, Wang Y, Bi Y, Liu G. The crosstalk between gut bacteria and host immunity in intestinal inflammation. J Cell Physiol. 2021;236(4):2239–2254. doi:10.1002/jcp.30024
16. Dore E, Boilard E. Bacterial extracellular vesicles and their interplay with the immune system. Pharmacol Ther. 2023;247:108443. doi:10.1016/j.pharmthera.2023.108443
17. Alabbas SY, Giri R, Oancea I, et al. Gut inflammation and adaptive immunity amplify Acetaminophen toxicity in bowel and liver. J Gastroenterol Hepatol. 2023;38(4):609–618. doi:10.1111/jgh.16102
18. Chung KS, Shin JS, Lee JH, et al. Protective effect of exopolysaccharide fraction from Bacillus subtilis against dextran sulfate sodium-induced colitis through maintenance of intestinal barrier and suppression of inflammatory responses. Int J Biol Macromol. 2021;178:363–372. doi:10.1016/j.ijbiomac.2021.02.186
19. Marcelino RC, Cardoso RM, Domingues E, Goncalves RV, Lima GDA, Novaes RD. The emerging risk of microplastics and nanoplastics on the microstructure and function of reproductive organs in mammals: a systematic review of preclinical evidence. Life Sci. 2022;295:120404. doi:10.1016/j.lfs.2022.120404
20. Lu H, Shen M, Chen Y, Yu Q, Chen T, Xie J. Alleviative effects of natural plant polysaccharides against DSS-induced ulcerative colitis via inhibiting inflammation and modulating gut microbiota. Food Res Int. 2023;167:112630. doi:10.1016/j.foodres.2023.112630
21. Granados-Martinez C, Alfageme-Lopez N, Navarro-Oviedo M, et al. Gut microbiota, bacterial translocation, and stroke: current knowledge and future directions. Biomedicines. 2024;12(12):2781. doi:10.3390/biomedicines12122781
22. Chen Y, Li H, Lai F, Min T, Wu H, Zhan Q. The influence and mechanisms of natural plant polysaccharides on intestinal microbiota-mediated metabolic disorders. Foods. 2024;13(23):3882. doi:10.3390/foods13233882
23. Dong S, Du Y, Wang H, Yuan W, Ai W, Liu L. Research progress on the interaction between intestinal flora and microRNA in pelvic inflammatory diseases. Noncoding RNA Res. 2025;11:303–312. doi:10.1016/j.ncrna.2025.01.007
24. Yang Y, Fan G, Lan J, Li X, Li X, Liu R. Polysaccharide-mediated modulation of gut microbiota in the treatment of liver diseases: promising approach with significant challenges. Int J Biol Macromol. 2024;280(Pt 1):135566. doi:10.1016/j.ijbiomac.2024.135566
25. Zhou Y, Liu X, Xu B. Research progress on the relationship between Parkinson’s disease and REM sleep behavior disorder. J Integr Neurosci. 2024;23(9):166. doi:10.31083/j.jin2309166
26. Hong X, Huang S, Jiang H, et al. Alcohol-related liver disease (ALD): current perspectives on pathogenesis, therapeutic strategies, and animal models. Front Pharmacol. 2024;15:1432480. doi:10.3389/fphar.2024.1432480
27. Zhou F, Lu Y, Sun T, et al. Antitumor effects of polysaccharides from Tetrastigma hemsleyanum Diels et Gilg via regulation of intestinal flora and enhancing immunomodulatory effects in vivo. Front Immunol. 2022;13:1009530. doi:10.3389/fimmu.2022.1009530
28. Jin Y, Liu H, Wang Y, et al. Pathogenesis and treatment of colitis-associated colorectal cancer: insights from traditional Chinese medicine. J Ethnopharmacol. 2025;338(Pt 2):119096.
29. Hou S, Yu J, Li Y, Zhao D, Zhang Z. Advances in fecal microbiota transplantation for gut dysbiosis-related diseases. Adv Sci. 2025;12(13):e2413197.
30. Tao SH, Lei YQ, Tan YM, Yang YB, Xie WN. Chinese herbal formula in the treatment of metabolic dysfunction-associated steatotic liver disease: current evidence and practice. Front Med Lausanne. 2024;11:1476419. doi:10.3389/fmed.2024.1476419
31. Noor S, Ali S, Summer M, et al. Therapeutic role of probiotics against environmental-induced hepatotoxicity: mechanisms, clinical perspectives, limitations, and future. Probiotics Antimicrob Proteins. 2025;17(2):516–540. doi:10.1007/s12602-024-10365-6
32. Zhu L, Guo J, Liu Q, et al. Lafutidine ameliorates indomethacin-induced small intestinal damage in rats by modifying the intestinal mucosal barrier, inflammation, and microbiota. Pharmacology. 2023;108(3):286–300. doi:10.1159/000529879
33. Kazmierczak-Siedlecka K, Daca A, Fic M, van de Wetering T, Folwarski M, Makarewicz W. Therapeutic methods of gut microbiota modification in colorectal cancer management - fecal microbiota transplantation, prebiotics, probiotics, and synbiotics. Gut Microbes. 2020;11(6):1518–1530. doi:10.1080/19490976.2020.1764309
34. Jahani-Sherafat S, Alebouyeh M, Moghim S, Ahmadi Amoli H, Ghasemian-Safaei H. Role of gut microbiota in the pathogenesis of colorectal cancer; a review article. Gastroenterol Hepatol Bed Bench. 2018;11(2):101–109.
35. Rokutan K, Kawahara T, Kuwano Y, Tominaga K, Nishida K, Teshima-Kondo S. Nox enzymes and oxidative stress in the immunopathology of the gastrointestinal tract. Semin Immunopathol. 2008;30(3):315–327. doi:10.1007/s00281-008-0124-5
36. Ehrlich AC, Patel S, Meillier A, Rothstein RD, Friedenberg FK. Chemoprevention of colorectal cancer in inflammatory bowel disease. Expert Rev Anticancer Ther. 2017;17(3):247–255. doi:10.1080/14737140.2017.1283987
37. Derikx LA, Kievit W, Drenth JP, et al. Prior colorectal neoplasia is associated with increased risk of ileoanal pouch neoplasia in patients with inflammatory bowel disease. Gastroenterology. 2014;146(1):119–28e1. doi:10.1053/j.gastro.2013.09.047
38. Sun D, Gong L, Wang X, Chen S, Yi J, Liu X. Pro-inflammatory cytokines promote the occurrence and development of colitis-associated colorectal cancer by inhibiting miR-615-5p. Inflamm Bowel Dis. 2023;29(12):1854–1864. doi:10.1093/ibd/izad105
39. Zhu M, Zhai Z, Wang Y, et al. Advancements in the application of artificial intelligence in the field of colorectal cancer. Front Oncol. 2025;15:1499223. doi:10.3389/fonc.2025.1499223
40. Horio Y, Uchino M, Igeta M, et al. Risk factors for the postoperative recurrence of ulcerative colitis-associated colorectal cancer. Int J Colorectal Dis. 2023;38(1):113. doi:10.1007/s00384-023-04410-z
41. Zhang M, Li X, Zhang Q, Yang J, Liu G. Roles of macrophages on ulcerative colitis and colitis-associated colorectal cancer. Front Immunol. 2023;14:1103617. doi:10.3389/fimmu.2023.1103617
42. Ruder EH, Laiyemo AO, Graubard BI, Hollenbeck AR, Schatzkin A, Cross AJ. Non-steroidal anti-inflammatory drugs and colorectal cancer risk in a large, prospective cohort. Am J Gastroenterol. 2011;106(7):1340–1350. doi:10.1038/ajg.2011.38
43. Tougeron D, Sha D, Manthravadi S, Sinicrope FA. Aspirin and colorectal cancer: back to the future. Clin Cancer Res. 2014;20(5):1087–1094. doi:10.1158/1078-0432.CCR-13-2563
44. Boland CR. Chronic inflammation, colorectal cancer and gene polymorphisms. Dig Dis. 2010;28(4–5):590–595. doi:10.1159/000320053
45. Hu W, Zheng Y, Xia P, Liang Z. The research progresses and future prospects of Tetrastigma hemsleyanum Diels et Gilg: a valuable Chinese herbal medicine. J Ethnopharmacol. 2021;271:113836. doi:10.1016/j.jep.2021.113836
46. Wang H, Wang A, Pu H, et al. Induction, flavonoids contents, and bioactivities analysis of hairy roots and true roots of Tetrastigma hemsleyanum Diels et Gilg. Molecules. 2023;28(6):2686.
47. Guo Z, Chen L, Liang X. Components research on Tetrastigma hemsleyanum Diels et Gilg: identification and effect of drying methods on the content of ten main constituents by targeting metabolomics method. J Pharm Biomed Anal. 2023;229:115375. doi:10.1016/j.jpba.2023.115375
48. Zhu R, Xu X, Ying J, Cao G, Wu X. The phytochemistry, pharmacology, and quality control of Tetrastigma hemsleyanum Diels & Gilg in China: a review. Front Pharmacol. 2020;11:550497. doi:10.3389/fphar.2020.550497
49. Yue E, Huang Y, Qian L, et al. Comparative analysis of proanthocyanidin metabolism and genes regulatory network in fresh leaves of two different ecotypes of Tetrastigma hemsleyanum. Plants. 2022;11(2). doi:10.3390/plants11020211
50. Ji T, Ji WW, Wang J, et al. A comprehensive review on traditional uses, chemical compositions, pharmacology properties and toxicology of Tetrastigma hemsleyanum. J Ethnopharmacol. 2021;264:113247. doi:10.1016/j.jep.2020.113247
51. Xia J, Li X, Lin M, et al. Screening out biomarkers of Tetrastigma hemsleyanum for anti-cancer and anti-inflammatory based on spectrum-effect relationship coupled with UPLC-Q-TOF-MS. Molecules. 2023;28(7):3021. doi:10.3390/molecules28073021
52. Sun L, Lu JJ, Wang BX, et al. Polysaccharides from Tetrastigma hemsleyanum Diels et Gilg: optimum extraction, monosaccharide compositions, and antioxidant activity. Prep Biochem Biotechnol. 2022;52(4):383–393. doi:10.1080/10826068.2021.1952600
53. Zhou F, Lin Y, Chen S, et al. Ameliorating role of Tetrastigma hemsleyanum polysaccharides in antibiotic-induced intestinal mucosal barrier dysfunction in mice based on microbiome and metabolome analyses. Int J Biol Macromol. 2023;241:124419. doi:10.1016/j.ijbiomac.2023.124419
54. Zhan L, Pu J, Zheng J, et al. Tetrastigma hemsleyanum Diels et Gilg ameliorates lipopolysaccharide induced sepsis via repairing the intestinal mucosal barrier. Biomed Pharmacother. 2022;148:112741. doi:10.1016/j.biopha.2022.112741
55. Lu J, Zhu B, Zhou F, et al. Polysaccharides from the aerial parts of Tetrastigma Hemsleyanum Diels et Gilg induce bidirectional immunity and ameliorate LPS-induced acute respiratory distress syndrome in mice. Front Pharmacol. 2022;13:838873. doi:10.3389/fphar.2022.838873
56. Wang B, Lin Y, Zhou M, et al. Polysaccharides from Tetrastigma Hemsleyanum Diels et Gilg attenuate LPS-induced acute lung injury by modulating TLR4/COX-2/NF-kappaB signaling pathway. Biomed Pharmacother. 2022;155:113755. doi:10.1016/j.biopha.2022.113755
57. Chu Q, Jia R, Chen M, et al. Tetrastigma hemsleyanum tubers polysaccharide ameliorates LPS-induced inflammation in macrophages and Caenorhabditis elegans. Int J Biol Macromol. 2019;141:611–621. doi:10.1016/j.ijbiomac.2019.09.039
58. Huang Q, He W, Khudoyberdiev I, Ye CL. Characterization of polysaccharides from Tetrastigma hemsleyanum Diels et Gilg Roots and their effects on antioxidant activity and H(2)O(2)-induced oxidative damage in RAW 264.7 cells. BMC Chem. 2021;15(1):9. doi:10.1186/s13065-021-00738-1
59. Zhou FM, Chen YC, Jin CY, et al. Polysaccharide isolated from Tetrastigma Hemsleyanum activates TLR4 in macrophage cell lines and enhances immune responses in OVA-immunized and LLC-bearing mouse models. Front Pharmacol. 2021;12:609059. doi:10.3389/fphar.2021.609059
60. Zhu B, Qian C, Zhou F, et al. Antipyretic and antitumor effects of a purified polysaccharide from aerial parts of Tetrastigma hemsleyanum. J Ethnopharmacol. 2020;253:112663. doi:10.1016/j.jep.2020.112663
61. Liu X, Liu X, Mao W, et al. Tetrastigma polysaccharide reprogramming of tumor-associated macrophages via PPARgamma signaling pathway to play antitumor activity in breast cancer. J Ethnopharmacol. 2023;314:116645. doi:10.1016/j.jep.2023.116645
62. Xiong Y, Wu X, Rao L. Tetrastigma hemsleyanum (Sanyeqing) root tuber extracts induces apoptosis in human cervical carcinoma HeLa cells. J Ethnopharmacol. 2015;165:46–53. doi:10.1016/j.jep.2015.02.030
63. Chen S, Luo M, Ma L, Lin W. Ethylacetate extract from Tetrastigma hemsleyanum inhibits proliferation and induces apoptosis in HepG2 and SMMC-7721 cells. Cancer Manag Res. 2018;10:3793–3799. doi:10.2147/CMAR.S168333
64. Xu H, Dai X, Hu X, et al. Phylogenetic analysis of R2R3-MYB family genes in Tetrastigma hemsleyanum Diels et Gilg and roles of ThMYB4 and ThMYB7 in flavonoid biosynthesis. Biomolecules. 2023;13(3):531. doi:10.3390/biom13030531
65. Bai Y, Jiang L, Li Z, Liu S, Hu X, Gao F. Flavonoid metabolism in Tetrastigma hemsleyanum Diels et Gilg based on metabolome analysis and transcriptome sequencing. Molecules. 2022;28(1):83. doi:10.3390/molecules28010083
66. Bai Y, Gu Y, Liu S, Jiang L, Han M, Geng D. Flavonoids metabolism and physiological response to ultraviolet treatments in Tetrastigma hemsleyanum Diels et Gilg. Front Plant Sci. 2022;13:926197. doi:10.3389/fpls.2022.926197
67. Han B, Zhai Y, Li X, et al. Total flavonoids of Tetrastigma hemsleyanum Diels et Gilg inhibits colorectal tumor growth by modulating gut microbiota and metabolites. Food Chem. 2023;410:135361. doi:10.1016/j.foodchem.2022.135361
68. Xia GS, Li SH, Zhou W. Isoquercitrin, ingredients in Tetrastigma hemsleyanum Diels et Gilg, inhibits hepatocyte growth factor/scatter factor-induced tumor cell migration and invasion. Cell Adh Migr. 2018;12(5):464–471. doi:10.1080/19336918.2018.1473664
69. Feng Z, Hao W, Lin X, Fan D, Zhou J. Antitumor activity of total flavonoids from Tetrastigma hemsleyanum Diels et Gilg is associated with the inhibition of regulatory T cells in mice. Onco Targets Ther. 2014;7:947–956. doi:10.2147/OTT.S61794
70. Sun Y, Guo F, Peng X, et al. Metabolism of phenolics of Tetrastigma hemsleyanum roots under in vitro digestion and colonic fermentation as well as their in vivo antioxidant activity in rats. Foods. 2021;10(9):2123. doi:10.3390/foods10092123
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.