Back to Journals » Journal of Inflammation Research » Volume 18
The Relationship Between Immune-Inflammatory Indexes and the Severity of Lower Urinary Tract Symptoms/Benign Prostatic Hyperplasia: A Cross-Sectional Study at a Tertiary Hospital in China
Authors Wu Y , Sheng J, Liu X, Huang Y, Zhang Y , Feng N
Received 7 March 2025
Accepted for publication 23 June 2025
Published 27 June 2025 Volume 2025:18 Pages 8509—8523
DOI https://doi.org/10.2147/JIR.S523193
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Wenjian Li
Yifan Wu,1,2,* Jiayi Sheng,1,* Xinwei Liu,1,2 Yongneng Huang,1,2 Yuwei Zhang,1,3 Ninghan Feng1,2
1Department of Urology, Jiangnan University Medical Center, Wuxi, Jiangsu Province, People’s Republic of China; 2Wuxi School of Medicine, Jiangnan University, Wuxi, Jiangsu Province, People’s Republic of China; 3Medical School of Nantong University, Nantong, Jiangsu Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ninghan Feng, Department of Urology, Jiangnan University Medical Center, 68 Zhongshan Road, Wuxi, 214002, People’s Republic of China, Tel +86 510 68562222, Email [email protected] Yuwei Zhang, Medical School of Nantong University, 9 Qiangyuan Road, Nantong, 226019, People’s Republic of China, Tel +86 513 85051875, Email [email protected]
Purpose: Lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH) are common urogenital system diseases in elderly men and can cause serious complications when the disease progresses to moderate and severe stages. Early and accurate identification is of great significance for prevention, treatment, and prognosis assessment. However, there is still a lack of effective and simple predictive indicators. This study aims to investigate whether immune-inflammatory markers derived from complete blood count (CBC) exhibit an independent association with the severity of BPH/LUTS.
Patients and Methods: This study included a total of 698 BPH/LUTS patients who met the inclusion criteria at the Department of Urology, Jiangnan University Medical Center. According to the International Prostate Symptom Score (IPSS) score, patients were divided into a mild group and a moderate-to-severe group. Binary logistic regression analysis was used to explore the association between the severity of BPH/LUTS and the neutrophil-to-lymphocyte ratio (NLR), systemic inflammatory response index (SIRI), and systemic immune-inflammatory index (SII).
Results: The median age of the participants was 70.00 (65.00, 75.00) years. After adjusting for confounding factors, the NLR, SIRI, and SII were positively correlated with the severity of BPH/LUTS. Compared with the lowest quartile, the highest quartile of NLR (OR = 6.20 [3.49– 11.02]), SIRI (OR = 7.49 [4.15– 13.50]), and SII (OR =7.85 [4.73– 16.61]) were most significantly associated with the risk of BPH/LUTS aggravation. In subgroups stratified by age, diet, physical activity, cardiovascular disease, and diabetes, NLR, SIRI, and SII were positively correlated with BPH/LUTS severity. In subgroups defined by waist circumference and dyslipidemia, SIRI and SII were positively correlated with BPH/LUTS severity. In the smoking subgroup, only SIRI showed a positive correlation with BPH/LUTS severity.
Conclusion: The findings suggest that NLR, SIRI, and SII are affordable and readily available detection methods that can be used as indicators for assessing the severity of BPH/LUTS.
Keywords: lower urinary tract symptoms, benign prostatic hyperplasia, neutrophil-to-lymphocyte ratio, systemic inflammatory response index, systemic immune-inflammatory index, cross-sectional study
Introduction
Benign prostatic hyperplasia (BPH) represents the most prevalent chronic and progressively worsening urological condition among elderly men, and it is also the primary cause of lower urinary tract symptoms (LUTS) in this demographic. Given China’s substantial elderly population, the overall prevalence rate of BPH/LUTS stands at 11.97%, significantly impacting patients’ quality of life.1 The management of BPH/LUTS encompasses watchful waiting, pharmacological therapy, and surgical intervention, with treatment selection primarily guided by the patient’s general condition, symptom severity, and associated complications. Therefore, monitoring symptom severity is crucial for developing high-quality comprehensive interventions and improving patient outcomes.
In addition to age and sex hormone levels, chronic inflammation is recognized as the third major factor contributing to the BPH/LUTS. Cytokines and growth factors released by inflammatory cells may stimulate the proliferation of stromal and epithelial cells.2 Several studies have reported that 80% of BPH patients exhibit concurrent chronic prostatic inflammation. Histological examination of specimens from prostatectomies due to BPH/LUTS commonly reveals inflammation of varying degrees in both the peripheral and transitional zones.3,4 Research has confirmed that C-reactive protein (CRP) and interleukin-8 (IL-8) are associated with the severity of BPH/LUTS, potentially serving as early screening and prognostic indicators.5–7 However, the cost and complexity of measuring these biomarkers limit their clinical application. In recent years, systemic inflammatory markers derived from complete blood counts have gained attention due to their low cost and ease of use. Numerous studies have found that systemic inflammatory markers or combinations thereof are associated with BPH, demonstrating predictive value for the progression and prognosis of BPH/LUTS.8–10 Ahmed et al demonstrated that that PLR, NLR and SII can be significantly employed for diagnosing BPH.11 These findings require replication in diverse ethnic populations, as current research predominantly focuses on European and American populations, with limited data on Asian populations. This study aims to explore the relationship between the neutrophil-to-lymphocyte ratio (NLR), systemic inflammation response index (SIRI), systemic immune-inflammation index (SII) and the severity of LUTS in elderly Chinese patients with BPH, understand the role of inflammation in the clinical progression of BPH, and thereby provide a reference basis for the early intervention and effect evaluation of patients.
Methods
Participants and Methods
We designed a cross-sectional observational study that was conducted between October 2023 and December 2024. A total of 698 patients diagnosed with BPH/LUTS were analyzed at the Urology Department of Jiangnan University Medical Center. Participants in this study must be aged between 60 and 80 years, exhibit one or more lower urinary tract symptoms, and have a confirmed diagnosis of BPH via digital rectal examination, color Doppler ultrasound of the urinary system, and computed tomography. Individuals with any of the following conditions will be excluded: infection or use of steroids or antibiotics within the past two weeks; prostate cancer; bladder tumors or other bladder diseases affecting normal urination function; stroke, Parkinson’s disease, or neurogenic bladder and other neurological disorders; severe cardiac, hepatic, or renal dysfunction; severe psychiatric illness; severe anxiety or depression; and a history of prostate or urethral surgery. The Ethics Committee of Jiangnan University Medical Center approved the study protocol, and each participant gave written informed consent. Figure 1 displays a flowchart of the participant selection process.
 |
Figure 1 Flowchart of the participant selection process. Abbreviations: BPH, benign prostatic hyperplasia; LUTS, lower urinary tract symptoms. |
Assessment and Grouping of BPH/LUTS
All participants were diagnosed with benign prostatic hyperplasia (BPH) through a comprehensive evaluation that included medical history, digital rectal examination, color Doppler ultrasound of the urinary system, and CT. The severity of LUTS was assessed using the International Prostate Symptom Score (IPSS), which is widely recognized as a key tool for evaluating LUTS severity in patients with BPH.12 The IPSS comprises seven items related to LUTS: incomplete bladder emptying, frequency, intermittency, urgency, weak stream, straining, and nocturia. Each item is scored on a scale from 0 to 5, yielding a total score ranging from 0 to 35. In this study, patients were divided into mild and moderate-to-severe groups based on IPSS scores, with the mild group having an IPSS < 8 and the moderate-to-severe group having an IPSS ≥ 8.
Definition of Immune-Inflammatory Indexes
Peripheral venous blood samples were collected on the second morning post-admission while patients were fasting. Automated haematology analyzers (Mindray Auto Hematology Analyzer BC6800) are used to measure lymphocyte, neutrophil, monocyte, and platelet count, which are presented as ×10^9/L. The immune-inflammatory indexes in our study were calculated as NLR=neutrophil/lymphocyte, SIRI=neutrophil× monocyte/lymphocyte, and SII=platelet ×neutrophil/lymphocyte.13 To elucidate the relationship between immunoinflammatory indexes and severity of BPH/LUTS, we classified each index into quartiles (Q1, Q2, Q3, Q4).
Assessment of Other Variables
Fasting blood glucose (FBG), triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), creatinine (Cr), total prostate-specific antigen (TPSA), and C-reactive protein (CRP) were all measured in fasting blood samples. In addition, electrocardiogram, prostate color Doppler ultrasound, and CT scans were performed on the second day of admission.
Potential Covariates
Our study incorporated three broad categories of covariates to analyze the relationship between inflammatory markers and LUTS. These categories include demographic characteristics, lifestyle factors, and chronic diseases. Demographic characteristics encompass age, education level, and marital status. Lifestyle factors comprise dietary habits, smoking status, alcohol consumption, physical activity, body mass index (BMI), and waist circumference. Smoking is defined as consuming at least one cigarette daily for over six months. Alcohol drinking is characterized by intake exceeding 25 grams per day. A healthy diet is operationalized as daily consumption of vegetables and fruits, regular intake of legumes (more than four days per week), weekly consumption of fish (more than one day per week), and limited red meat intake (less than seven days per week). Active physical activity is defined as engaging in physical activity at least three times per week. General obesity is indicated by a BMI of 28 kg/m² or higher, while central obesity is denoted by a waist circumference of 90cm or more. Chronic diseases are those diagnosed within the past year by professional medical institutions, including hypertension, diabetes, cardiovascular disease (CVD), hyperlipidemia, chronic lung disease, and cerebrovascular disease.
Statistical Analysis
Statistical analyses were performed using R Studio (version 4.4.2). Continuous variables following a normal distribution were shown as mean ± standard error, while those without a normal distribution were indicated by median (P25, P75). Categorical variables were displayed as percentages. Non-parametric tests were employed for between-group comparisons of non-normally distributed continuous variables. Chi-square tests were utilized for categorical variables. Binary logistic regression analysis was conducted to examine the association between the systemic inflammatory response index and the severity of BPH/ LUTS. Three models were used for the analysis: (1) The crude model had no covariates; (2) Model 1, which adjusted for age, diet, BMI, waist circumference, physical activity, and smoking status; and (3) Model 2, which further adjusted for hypertension, CVD, dyslipidemia, and diabetes on top of the factors in Model 1. Additionally, trend testing was performed using logistic regression based on the severity of LUTS. Smooth curve fitting was applied to investigate potential linear or nonlinear relationships between NLR, SIRI, SII, and LUTS. Subgroup and interaction analyses were conducted based on age, diet, waist circumference, physical activity, smoking status, CVD, dyslipidemia, and diabetes. A two-tailed p-value < 0.05 was considered statistically significant for differences.
Results
Characteristics of Study Subjects
A total of 698 participants were included in our study, comprising 200 individuals in the mild group and 498 in the moderate-to-severe group (the detailed characteristics were presented in Table 1). Compared with the mild group, participants in the moderate-to-severe group exhibited higher mean age (70.80 ± 7.86y vs 67.90 ± 7.26y), BMI (24.69 ± 2.72 kg/m² vs 23.83 ± 3.51 kg/m²), and waist circumference (91.81 ± 6.38 cm vs 89.09 ± 4.68 cm). Additionally, a greater proportion of participants in the moderate-to-severe group reported unhealthy diets (43.23% vs 31.63%), smoking (33.27% vs 20.41%), and lack of physical activity (25.50% vs 14.80%). Furthermore, the prevalence of chronic diseases was higher in the moderate-to-severe group, including diabetes (25.50% vs 14.80%) and hyperlipidemia (25.30% vs 16.84%). The values for NLR, SIRI, SII, neutrophil count, monocyte count, and platelet count, were also significantly elevated in the moderate-to-severe group compared to the mild group. Other than that, there were no notable differences between the groups.
 |
Table 1 Baseline Characteristics of Participants |
Association Between Systemic Inflammatory Response Indexes and Severity of BPH/LUTS
We conducted a multivariate logistic regression analysis with the Q1 group as the reference (Table 2). The results showed that the median, upper quartile, and lower quartile values for NLR, SIRI, and SII were 2.40 (1.71, 3.48), 0.88 (0.60, 1.41), and 430.50 (288.91, 673.53), respectively. In the unadjusted model, BPH/LUTS severity was positively associated with NLR, SIRI, and SII. After adjusting for potential confounders including age, diet, BMI, waist circumference, physical activity, and smoking status, the associations remained statistically significant. Specifically, Model 2 revealed that the odds ratios (OR) and 95% confidence intervals (CI) for the Q4 group were as follows: NLR (OR = 6.20[3.49, 11.02]), SIRI (OR = 7.49 [4.15, 13.50]), and SII (OR =7.85[4.28, 14.42]). Similar trends were observed in the Q2 and Q3 groups for NLR and SIRI, and in the Q3 group for SII. Trend tests for these associations were statistically significant, with all P-values <0.001. Smooth curve fitting was subsequently employed to illustrate the trends in the relationship between NLR, SIRI, SII, and the risk of exacerbation of LUTS. The results indicated that higher levels of NLR, SIRI, and SII were associated with more severe LUTS (Figure 2).
 |
Table 2 OR (95% CI) for LUTS Across Quartiles of NLR, SIRI, and SII |
Subgroup Analysis
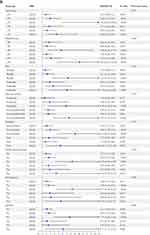
Subgroup analyses were performed to evaluate the potential modifications of the association between systemic inflammatory response indexes and BPH/LUTS severity in fully adjusted Model 2, where stratification factors were not included. As shown in Figure 3, we observed a positive association between NLR, SIRI, SII, and BPH/LUTS severity across subgroups defined by age, diet, physical activity, CVD, and diabetes. In the waist circumference and dyslipidemia subgroups, SIRI and SII exhibited a positive correlation with BPH/LUTS severity. In the smoking subgroup, only SIRI showed a positive correlation with BPH/LUTS severity. Taking the Q1 group as the reference, we observed that the positive correlation between the highest quartile of the aforementioned immune-inflammatory indicators and the risk of BPH/LUTS was the most pronounced. Additionally, interaction effects were consistent across all subgroups, with all p-values for interactions > 0.05. Figure 3 Continued. Figure 3 Continued.
Discussion
An increasing body of evidence suggests that benign prostatic hyperplasia (BPH) is an immune-mediated inflammatory condition, wherein inflammation significantly contributes to disease progression and pathogenesis. Nearly all surgical specimens from BPH patients exhibit inflammatory cell infiltration, predominantly comprising lymphocytes, macrophages, and mast cells. Additionally, these patients tend to have higher IPSS scores and an elevated risk of complications.14–16 Based on this, non-steroidal anti-inflammatory drugs(NSAIDs) have been incorporated into the standard drug treatment for BPH to prevent the deterioration of the condition and symptoms, and have achieved certain therapeutic effects.17,18 Therefore, immune-inflammatory markers are anticipated to serve as biomarkers for evaluating disease severity and predicting prognosis. Prostate biopsy is an invasive and traumatic procedure, rendering it unsuitable for population-wide screening or the monitoring of disease progression and treatment efficacy. Furthermore, patient acceptance of this procedure is relatively low. In recent years, biomarkers such as interleukin-8 (IL-8) in seminal plasma, inducible T-cell co-stimulatory molecule in urine, and simplified serum markers including C-reactive protein (CRP) and interleukin-6 (IL-6) have been utilized to predict the prognosis of BPH/LUTS. However, some of these biomarkers are expensive and difficult to obtain, while others have limited predictive power.19–22
NLR, SIRI, and SII represent novel composite inflammatory markers derived from the complete blood cell count. These indexes integrate parameters such as lymphocytes, neutrophils, monocytes, and platelets, offering a more cost-effective, comprehensive, and accessible alternative to traditional inflammatory biomarkers. They provide valuable insights into the equilibrium between immune and inflammatory responses within the human body. Recently, NLR, SIRI, and SII have been introduced as potential biomarkers for evaluating inflammation and predicting the prognosis of prostate cancer and other diseases,23–25 but there are few studies on their relationship with the risk of BPH/LUTS. Data from some studies are derived from public databases, while others focus solely on a single inflammatory immune indicator. To explore the relationship between immune- inflammatory markers and the severity of BPH/LUTS, we conducted a cross-sectional study involving 698 elderly male inpatients with BPH/LUTS at a tertiary hospital in China. The findings revealed that the levels of NLR, SIRI, and SII were significantly elevated in patients with moderate to severe LUTS. After adjusting for potential confounding factors, the levels of NLR, SIRI and SII were still positively correlated with the severity of LUTS. These findings align with most existing literature on the topic. As reported by Liu Ming et al, in a study of 2709 male patients over the age of 40 from the NHANES cohort, the SII, SIRI, and NLR demonstrated positive correlations with LUTS.8 Ozer et al demonstrated that NLR was positively associated with both the severity and progression of benign prostatic hyperplasia (BPH).26 Horsanali et al found that elevated SII levels were significantly linked to the progression of LUTS and poorer outcomes in men with BPH.10 Notably, our study identified a linear relationship between NLR, SIRI, and SII levels and the risk of exacerbation of LUTS. The severity of LUTS increased in tandem with rising NLR, SIRI, and SII levels. Overall, these results suggest that NLR, SIRI, and SII may serve as valuable clinical indicators for monitoring the progression and prognosis of BPH/LUTS.
The potential causes of prostatitis include infection (bacterial or viral), hormonal changes, dietary or environmental factors, autoimmune response, urinary reflux inside prostate collecting ducts, and systemic inflammation associated with metabolic syndrome (MetS).27 The exact mechanism by which NLR, SIRI, and SII assess the risk of LUTS remains unclear, but it may be attributed to the pathways through which oxidative stress, inflammatory mediators, and insulin-like growth factors promote non-malignant and unregulated prostate growth. Research has found that stimulation by exogenous or endogenous antigens may induce the prostate to initiate an inflammatory response. Prostatic stromal cells act as targets for infection factors by activating Toll-like receptors (TLRs), and then as antigen-presenting cells, which activate the immune responses of T cells and macrophages, releasing more inflammatory factors and chemokines (such as IL-6, IL-8, etc). They respond to cytokines interferon-γ, IL-2 and IL-17 by increasing the production of IL-8 and IL-6, leading to excessive growth of epithelial cells and stromal cells.28,29 In addition, inflammatory cytokines can prolong the chronic immune response in BPH and induce the growth of prostatic fibromuscular tissue through autocrine or paracrine loops or by inducing COX-2 expression. COX-2 can stimulate the production of prostaglandins, induce the proliferation of bladder smooth muscle cells, and lead to bladder outlet obstruction.30,31 This study also found that both SIRI and SII had higher OR values than NLR. The possible reason is that, in addition to the components of neutrophils and lymphocytes, SIRI and SII respectively incorporate monocytes and platelets. Monocytes can activate T lymphocytes through antigen presentation, further triggering adaptive immune responses. Platelets are known as effectors of inflammatory responses. After activation, they adhere to the surfaces of neutrophils and monocytes and interact with lymphocytes.32,33 Subgroup analysis revealed that age, diet, smoking, waist circumference, physical activity, coronary heart disease, hyperlipidemia, and diabetes subgroups respectively demonstrated a positive correlation with LUTS risk at different levels of NLR, SIRI and SII, and all showed the strongest association at the Q4 quartile level, which is consistent with clinical and epidemiological studies. According to research, nicotine may increase the activity of the sympathetic nervous system, causing urinary retention symptoms by increasing the tension of the bladder smooth muscle. Additionally, studies have found that in BPH patients who smoke, the levels of serum malondialdehyde and interleukin-8 are higher.34,35 Adhering to a Mediterranean diet can reduce the risk of BPH, while vitamin D deficiency can activate the NF-κb/IL-6 pathway and upregulate the stat3-mediated pathway that stimulates cell proliferation and growth, thereby inducing prostatic inflammation and fibrosis.36 A large prospective study found that men with the highest levels of physical activity were 19% less likely to develop moderate or more severe LUTS than those with the lowest levels of physical activity.37 Metabolic syndrome is a complex cluster of metabolic disorders, including obesity, hyperglycemia, hyperlipidemia, and hypertension.38 There is considerable evidence suggesting that patients with metabolic syndrome have elevated levels of interleukin (IL). Obese men with lower urinary tract symptoms/benign prostatic hyperplasia who have elevated insulin resistance or suffer from hypertension and hypercholesterolemia may experience persistent prostatic inflammation. Metabolic syndrome may be an independent risk factor for prostatic inflammation and fibrosis.39,40 Therefore, closely monitoring the inflammatory levels of these individuals with LUTS is of greater significance.
In comparison to earlier studies, the current study has several significant advantages. First, all participants in this study were from clinical populations, making the data more reliable. Second, after adjusting for multiple potential confounding variables, the results remained statistically significant, indicating that increased NLR, SIRI, and SII levels are independently associated with a higher risk of worsening BPH/LUTS. Additionally, we also explored the possible biological mechanisms of inflammation in the occurrence and development of benign prostatic hyperplasia, providing a theoretical basis for the clinical promotion of immune-inflammatory indicators in disease risk assessment and prognosis prediction. It goes without saying that the current study also has several limitations. First, it is a single-center study, which may lead to bias in participant selection. Second, it is a cross-sectional study, so no definitive causal inferences can be drawn regarding the findings. Third, although adjustments were made for certain covariates, key potential confounding factors such as socioeconomic status and prostate volume were not accounted for, which may have introduced bias into the research findings. Fourth, to avoid missing some potentially relevant variables, we did not adjust for multiple comparisons, which may increase the risk of false positives. Therefore, the next step should be a multicenter, large-scale prospective study that fully accounts for the influence of key potential confounders. Such a study would not only serve to validate the reliability of the current research conclusions but also facilitate more in-depth exploration of the relationship between the immune-inflammation index and a broader spectrum of academic disciplines, thereby enhancing its applicability and universality across diverse contexts.
Conclusion
In summary, our study indicates that higher levels of NLR, SIRI, and SII may be associated with more severe LUTS in BPH patients. We speculate that NLR, SIRI, and SII can serve as non-invasive and easily accessible composite biomarkers for identifying the severity of BPH/LUTS and monitoring disease progression, which holds significant value for the primary and secondary prevention of BPH in middle-aged and elderly men.
Abbreviations
BPH, benign prostatic hyperplasia; LUTS, lower urinary tract symptoms; CVD, cardiovascular disease; BMI, body mass index; Neu, neutrophil; Lym, lymphocyte; Mono, monocyte; PLT, platelet; NLR, neutrophil-to-lymphocyte ratio; SIRI, systemic inflammation response index; SII, systemic immune-inflammation index.
Data Sharing Statement
The datasets used and/or analyzed in the current study will be available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study received ethical approval from the Ethics Committee of Jiangnan University Medical Center (No: 2021-Y-3). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. According to the declaration of Helsinki, all participants signed an informed consent form before participating in the study.
Acknowledgments
The authors thank the participating volunteers.
Author Contributions
All authors made a significant contribution to the work reported, whether that be in study design, execution, data acquisition and analysis, or in all of these areas; participated in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agree to take responsibility for all aspects of the work.
Funding
This study was supported by Wuxi “key medical discipline construction” Municipal Clinical Medical Center Project (LCZX2021003) and the National Natural Science Foundation of China (Item No: 82370777).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Xiong Y, Zhang Y, Li X, et al. The prevalence and associated factors of lower urinary tract symptoms suggestive of benign prostatic hyperplasia in aging males. Aging Male. 2020;23(5):1432–1439. doi:10.1080/13685538.2020.1781806
2. Fibbi B, Penna G, Morelli A, et al. Chronic inflammation in the pathogenesis of benign prostatic hyperplasia. Int J Androl. 2010;33(3):475–488. doi:10.1111/j.1365-2605.2009.00972.x
3. Di Silverio F, Gentile V, De Matteis A, et al. Distribution of inflammation, pre-malignant lesions, incidental carcinoma in histologically confirmed benign prostatic hyperplasia: a retrospective analysis. Eur Urol. 2003;43(2):164–175. doi:10.1016/s0302-2838(02)00548-1
4. De Nunzio C, Salonia A, Gacci M, et al. Inflammation is a target of medical treatment for lower urinary tract symptoms associated with benign prostatic hyperplasia. World J Urol. 2020;38(11):2771–2779. doi:10.1007/s00345-020-03106-1
5. Kim JH, Doo SW, Yang WJ, et al. Association between high-sensitivity C-reactive protein and lower urinary tract symptoms in healthy Korean populations. Urology. 2015;86(1):139–144. doi:10.1016/j.urology.2015.03.035
6. St Sauver JL, Sarma AV, Jacobson DJ, et al. Associations between C-reactive protein and benign prostatic hyperplasia/lower urinary tract symptom outcomes in a population-based cohort. Am J Epidemiol. 2009;169(11):1281–1290. doi:10.1093/aje/kwp085
7. Snyper NYF, Pike J, Ekwueme K, et al. Selective biomarkers for inflammation and infection are associated with post-operative complications following transperineal template prostate biopsy (TTPB): a single-centre observational clinical pilot-study. Eur J Med Res. 2022;27(1):187. doi:10.1186/s40001-022-00807-8
8. Liu W, Wang J, Wang M, et al. Association between immune-inflammatory indexes and lower urinary tract symptoms: an analysis of cross-sectional data from the US National Health and Nutrition Examination Survey (2005-2008). BMJ Open. 2024;14(3):e080826. doi:10.1136/bmjopen-2023-080826
9. Shi C, Cao H, Zeng G, et al. The relationship between complete blood cell count-derived inflammatory biomarkers and benign prostatic hyperplasia in middle-aged and elderly individuals in the United States: evidence from NHANES 2001-2008. PLoS One. 2024;19(7):e0306860. doi:10.1371/journal.pone.0306860
10. Horsanali MO, Dil E, Caglayan A, et al. The predictive value of the systemic immune-inflammation index for the progression of lower urinary tract symptoms in men. Asian Pac J Cancer Prev. 2023;24(11):3845–3850. doi:10.31557/APJCP.2023.24.11.3845
11. Ahmed R, Hamdy O, Awad RM. Diagnostic efficacy of systemic immune-inflammation biomarkers in benign prostatic hyperplasia using receiver operating characteristic and artificial neural network. Sci Rep. 2023;13(1):14801. doi:10.1038/s41598-023-41781-3
12. Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association Symptom Index for Benign Prostatic Hyperplasia. J Urol. 2017;197(2S):S189–S197. doi:10.1016/j.juro.2016.10.071
13. Zhou D, Yang H, Zeng L, et al. Calculated inflammatory markers derived from complete blood count results, along with routine laboratory and clinical data, predict treatment failure of acute peritonitis in chronic peritoneal dialysis patients. Ren Fail. 2023;45(1):2179856. doi:10.1080/0886022X.2023.2179856
14. Meng Y, Yu W, Liu Z, et al. The inflammation patterns of different inflammatory cells in histological structures of hyperplasic prostatic tissues. Transl Androl Urol. 2020;9(4):1639–1649. doi:10.21037/tau-20-448
15. Hu J, Zhang L, Zou L, et al. Role of inflammation in benign prostatic hyperplasia development among Han Chinese: a population-based and single-institutional analysis. Int J Urol. 2015;22(12):1138–1142. doi:10.1111/iju.12914
16. Cakir SS, Polat EC, Ozcan L, et al. The effect of prostatic inflammation on clinical outcomes in patients with benign prostate hyperplasia. Prostate Int. 2018;6(2):71–74. doi:10.1016/j.prnil.2017.12.003
17. St Sauver JL, Jacobsen SJ, Jacobson DJ, et al. Statin use and decreased risk of benign prostatic enlargement and lower urinary tract symptoms. BJU Int. 2011;107(3):443–450. doi:10.1111/j.1464-410X.2010.09598.x
18. Kaplan SA. Re: non-steroidal anti-inflammatory drugs for lower urinary tract symptoms in benign prostatic hyperplasia: systematic review and meta-analysis of randomized controlled trials. J Urol. 2013;190(5):1819. doi:10.1016/j.juro.2013.07.048
19. Penna G, Mondaini N, Amuchastegui S, et al. Seminal plasma cytokines and chemokines in prostate inflammation: interleukin 8 as a predictive biomarker in chronic prostatitis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur Urol. 2007;51(2):
20. Liu L, Li Q, Han P, et al. Evaluation of interleukin-8 in expressed prostatic secretion as a reliable biomarker of inflammation in benign prostatic hyperplasia. Urology. 2009;74(2):340–344. doi:10.1016/j.urology.2009.02.064
21. Inamura S, Ito H, Shinagawa T, et al. Serum C-reactive protein level is not associated with prostatic inflammation but with overactive detrusor in patients with benign prostatic hyperplasia. Neurourol Urodyn. 2019;38(6):1728–1736. doi:10.1002/nau.24051
22. Robert G, Descazeaud A, Nicolaiew N, et al. Inflammation in benign prostatic hyperplasia: a 282 patients’ immunohistochemical analysis. Prostate. 2009;69(16):1774–1780. doi:10.1002/pros.21027
23. Meng L, Yang Y, Hu X, et al. Prognostic value of the pretreatment systemic immune-inflammation index in patients with prostate cancer: a systematic review and meta-analysis. J Transl Med. 2023;21(1):79. doi:10.1186/s12967-023-03924-y
24. Yilmaz H, Cinar NB, Avci IE, et al. The systemic inflammation response index: an independent predictive factor for survival outcomes of bladder cancer stronger than other inflammatory markers. Urol Oncol. 2023;41(5):256e1–256e8. doi:10.1016/j.urolonc.2022.11.011
25. Song Y, Gu Y, Guo H, et al. Association between neutrophil-to-lymphocyte ratio and benign prostatic hyperplasia: results from the TCLSIH cohort study. J Inflamm Res. 2023;16:4857–4866. doi:10.2147/JIR.S431049
26. Ozer K, Horsanali MO, Gorgel SN, et al. Association between benign prostatic hyperplasia and neutrophil-lymphocyte ratio, an indicator of inflammation and metabolic syndrome. Urol Int. 2017;98(4):466–471. doi:10.1159/000448289
27. Ficarra V, Sekulovic S, Zattoni F, et al. Why and how to evaluate chronic prostatic inflammation. Eur Urol Suppl. 2013;12(5):110–115. doi:10.1016/j.eursup.2013.08.002
28. De Nunzio C, Kramer G, Marberger M, et al. The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. Eur Urol. 2011;60(1):106–117. doi:10.1016/j.eururo.2011.03.055
29. Kramer G, Marberger M. Could inflammation be a key component in the progression of benign prostatic hyperplasia? Curr Opin Urol. 2006;16(1):25–29. doi:10.1097/01.mou.0000193368.91823.1b
30. Huang L, LaBonte MJ, Craig SG, et al. Inflammation and prostate cancer: a multidisciplinary approach to identifying opportunities for treatment and prevention. Cancers. 2022;14(6). doi:10.3390/cancers14061367
31. Chen KC, Peng CH, Wang HE, et al. Modeling of the pH- and the temperature-dependent deviations of the free to total PSA (prostate specific antigen) ratios for clinical predictability of prostate cancer and benign prostate hyperplasia. Bull Math Biol. 2004;66(3):423–445. doi:10.1016/j.bulm.2003.08.014
32. Elewaut A, Estivill G, Bayerl F, et al. Cancer cells impair monocyte-mediated T cell stimulation to evade immunity. Nature. 2025;637(8046):716–725. doi:10.1038/s41586-024-08257-4
33. Chen Y, Zhong H, Zhao Y, et al. Role of platelet biomarkers in inflammatory response. Biomark Res. 2020;8(1):28. doi:10.1186/s40364-020-00207-2
34. Al-Barzinj RMGT. Estimation levels of prostate-specific antigen, interleukin-8, oxidative stress and some inflammatory markers in sera of benign prostatic hyperplasia patients who have smoking habits as a risk factor. Cell Mol Biol. 2020;66(7):124–130. doi:10.14715/cmb/2020.66.7.19
35. Rohrmann S, Crespo CJ, Weber JR, et al. Association of cigarette smoking, alcohol consumption and physical activity with lower urinary tract symptoms in older American men: findings from the third national health and nutrition examination survey. BJU Int. 2005;96(1):77–82. doi:10.1111/j.1464-410X.2005.05571.x
36. Zhang ZH, Luo B, Xu S, et al. Vitamin D deficiency promotes prostatic hyperplasia in middle-age mice through exacerbating local inflammation. J Steroid Biochem Mol Biol. 2018;182:14–20. doi:10.1016/j.jsbmb.2018.04.006
37. Mondul AM, Giovannucci E, Platz EA. A prospective study of physical activity, sedentary behavior, and incidence and progression of lower urinary tract symptoms. J Gen Intern Med. 2020;35(8):2281–2288. doi:10.1007/s11606-020-05814-1
38. Cantiello F, Cicione A, Salonia A, et al. Metabolic syndrome correlates with peri-urethral fibrosis secondary to chronic prostate inflammation: evidence of a link in a cohort of patients undergoing radical prostatectomy. Int J Urol. 2014;21(3):264–269. doi:10.1111/iju.12233
39. Fowke JH, Koyama T, Fadare O, et al. Does inflammation mediate the obesity and bph relationship? An epidemiologic analysis of body composition and inflammatory markers in blood, urine, and prostate tissue, and the relationship with prostate enlargement and lower urinary tract symptoms. PLoS One. 2016;11(6):e0156918. doi:10.1371/journal.pone.0156918
40. He Q, Wang Z, Liu G, et al. Metabolic syndrome, inflammation and lower urinary tract symptoms: possible translational links. Prostate Cancer Prostatic Dis. 2016;19(1):7–13. doi:10.1038/pcan.2015.43
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
The P.R.OS.T.A.T.E Nomogram for the Preoperative Prediction of Clinical Efficacy of Transurethral Resection of the Prostate in Benign Prostatic Hyperplasia Patients
Tian Y, Zhang H, Cao Y, Yang L, Luo G
Clinical Interventions in Aging 2022, 17:845-855
Published Date: 23 May 2022
Correlation Between Benign Prostatic Hyperplasia/Lower Urinary Tract Symptoms and Renal Function in Elderly Men Aged 80 Years and Older
Wang Q, Zhang B, Li B, Yang S, Wang Z, Han C, Wu J, Tian R
Clinical Interventions in Aging 2023, 18:61-69
Published Date: 13 January 2023
The Association of Pretreatment Systemic Immune Inflammatory Response Index (SII) and Neutrophil-to-Lymphocyte Ratio (NLR) with Lymph Node Metastasis in Patients with Papillary Thyroid Carcinoma
Gu Y, Yu M, Deng J, Lai Y
International Journal of General Medicine 2024, 17:2887-2897
Published Date: 1 July 2024



