Back to Journals » Journal of Inflammation Research » Volume 18
The Role and Mechanism of Protein Post‑Translational Modification in Rheumatoid Arthritis
Authors Wen J, Liu J , Wan L, Wang F
Received 15 March 2025
Accepted for publication 2 July 2025
Published 11 July 2025 Volume 2025:18 Pages 9055—9078
DOI https://doi.org/10.2147/JIR.S528487
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ujjwol Risal
Jianting Wen,1– 3 Jian Liu,1– 3 Lei Wan,1– 3 Fanfan Wang2,3
1Department of Rheumatology and Immunology, First Affiliated Hospital of Anhui University of Chinese Medicine, Hefei, Anhui Province, 230031, People’s Republic of China; 2Institute of Rheumatology, Anhui Academy of Chinese Medicine, Hefei, Anhui Province, 230012, People’s Republic of China; 3Anhui Province Key Laboratory of Modern Chinese Medicine Department of Internal Medicine Application Foundation Research and Development, Hefei, Anhui, 230031, People’s Republic of China
Correspondence: Jian Liu, Department of Rheumatology and Immunology, First Affiliated Hospital of Anhui University of Chinese Medicine, Hefei, Anhui Province, 230031, People’s Republic of China, Tel +86 0551 62838582, Fax +86 0551 62821605, Email [email protected]
Abstract: Rheumatoid arthritis (RA) is a chronic autoimmune disease that significantly compromises patient quality of life due to its high prevalence and risk of disability. While its etiology remains incompletely understood, increasing evidence highlights the critical involvement of epigenetic mechanisms, particularly post-translational modifications (PTMs), in RA pathogenesis. Advances in proteomics have identified various PTMs—including phosphorylation, methylation, acetylation, ubiquitination, glycosylation, lactylation, as well as citrullination and carbamylation—as key regulators of inflammation, immune response, and tissue remodeling in RA. Importantly, dysregulated PTMs may alter protein structure and function, thereby contributing to disease progression. This review systematically summarizes current knowledge on the roles and mechanisms of major PTMs in RA, with a special focus on the cross-talk between PTMs, their interaction with non-coding RNAs, and the emerging therapeutic potential of traditional Chinese medicine (TCM) targeting PTMs. These insights may provide novel perspectives for the diagnosis and treatment of RA.
Keywords: protein post-translational modification, rheumatoid arthritis, methylation, acetylation, lactylation
Introduction
Rheumatoid arthritis (RA) is a common chronic autoimmune disease that primarily affects joints and can lead to joint destruction and deformity, with a global prevalence of approximately 0.5%-1.0%.1,2 RA not only predominantly affects the joints but also affects extra-articular structures (including the skin, heart, and lung) and gastrointestinal, neurologic, and vascular systems.3,4 More seriously, RA can easily cause disability, which seriously affects the quality of life of patients.5 Currently, the exact pathogenesis of RA has not been fully elucidated. Most researchers have supported that multiple factors contribute to the onset of RA, including genetics, immunity, endocrine disorders, environment, smoking, infection, etc.6,7 Additionally, a recent study has pointed out that epigenetics may play a significant role in the development of RA.8 Epigenetics involves multiple mechanisms [such as DNA methylation, histone modification, and non-coding RNA (ncRNA) regulation], which are closely related to RA onset and progression.
The complexity and diverse functions of the human proteome largely depend on PTMs. PTMs refer to the modifications of proteins after their biosynthesis through covalent binding of specific chemical groups. These modifications not only affect the folding and conformation of proteins but also significantly alter their activity and function. For example, it has been shown that phosphorylation, acetylation, glycosylation—as well as citrullination and carbamylation—are common post-translational modifications that play crucial roles in regulating protein structure, function, and immunogenicity.9 In recent years, with the advancement of mass spectrometry (MS) technology, researchers have been able to comprehensively characterize PTMs at the proteomic level. These technological advancements not only reveal the broad functional roles of different modification types but also drive research into the origins and evolution of regulatory enzymes. Additionally, the regulatory role of PTMs in protein-protein interactions has gradually emerged. The PTMcode v2 database is a specialized resource for describing the regulatory role of PTMs in protein interactions, which can be used to predict their functional associations through methods such as residue co-evolution and proximity.10 To date, with advanced detection technologies, over 600 PTMs of proteins have been identified. These PTMs play crucial roles in enzyme function and assembly, receptor activation, protein interactions, protein localization, cell metabolism, signaling pathways, etc. Emerging research has highlighted that aberrant PTMs are tightly implicated in diverse human diseases, including RA.
Considering the crucial role of PTMs in RA, it’s of great significance to deeply understand how PTMs influence the pathogenesis of RA, which is essential for developing effective preventive and therapeutic strategies. The present study systematically reviewed and profiled the most recent advances in the roles of PTMs in RA, as well as the therapeutic effects of traditional Chinese medicine (TCM) on RA by regulating PTMs. This review may provide a new basis and direction for studying RA diagnosis and treatment from the perspective of protein modification.
Types of Protein PTMs and Their Biological Functions
Transcription, translation, and PTMs form a multi-layered dynamic network that is crucial for the biochemical and physiological diversity and complexity observed in living organisms. The dynamic and reversible nature of PTMs allows for the precise regulation of cellular processes and signal transduction. A key aspect of PTMs is their ability to rapidly alter protein activity, localization, and interactions, thereby modulating signal transduction pathways.11
PTMs further add to this complexity by altering protein function, localization, and interaction capabilities.12,13 PTMs (such as phosphorylation, methylation, and acetylation) can modulate the activity of proteins involved in transcriptional and post-transcriptional regulation, thus affecting gene expression outcomes (Figure 1). The interplay between different PTMs, commonly referred to as PTM crosstalk, can provide additional layers of regulation that allow for fine-tuning of cellular processes. This intricate network of transcription, translation, and PTMs underscores the sophisticated regulatory mechanisms that underpin cellular diversity and adaptability.14
 |
Figure 1 Common type of protein PTMs (by figdraw). |
Phosphorylation is the first type of identified PTM discovered, then it takes more than one hundred years to discover the latest lactylation. The advancements in detection technology, especially the rapid development of MS, have provided researchers with unprecedented opportunities to explore the biological functions of novel PTMs.15 This will not only help reveal the molecular mechanism of diseases, but also provide an important scientific basis for the development of new therapies. The principles and mechanisms of the most common PTMs are described below.
Phosphorylation
Phosphorylation, as an important PM of proteins, was first discovered in 1906.16 In mammals, approximately 30% of proteins undergo phosphorylation, a process dynamically regulated by protein kinases and phosphatases that has significant impacts on many cellular activities. Phosphorylation not only participates in membrane transport but also plays a crucial role in cell signaling, proliferation, differentiation, and apoptosis.17 Protein phosphorylation is a crucial mechanism in cellular signaling, involving the transfer of the γ-phosphate group from ATP to specific amino acid residues in substrate proteins.
Phosphorylation is one of the most extensively studied PTMs and tightly participates in regulating various cellular processes. The significance of phosphorylation is underscored by its involvement in signal transduction pathways, where it acts as a molecular switch to turn on or off protein activity. This modification is catalyzed by kinases, which transfer a phosphate group from ATP to specific amino acids on target proteins, typically serine, threonine, or tyrosine residues. The dynamic nature of phosphorylation allows cells to respond rapidly to external stimuli, making it a key player in cellular communication and regulation.18 Moreover, in addition to common serine, threonine, or tyrosine residues, protein phosphorylation also includes biologically important phosphorylated amino acids (such as phospho-aspartic acid and phospho-glutamic acid), which play significant roles in signaling pathways.
In the study of protein phosphorylation, identifying substrates of protein kinases is crucial for understanding cellular processes. In recent years, with the development of MS technology, a growing number of phosphorylation sites have been identified, providing a foundation for constructing phosphorylation networks between protein kinases and their substrates. However, despite significant progress, the inference of phosphorylation sites and identification of related kinases remain unresolved issues in the biology of protein kinases.
Methylation
Protein methylation is an important biochemical modification process that involves transferring a methyl group from an active methyl compound to amino acid residues of a protein. In 1959, this process was first reported in bacteria, involving methylation of flagellar proteins.19 Methylation has a wide range of functions and significance within organisms. At the end of the 20th century, with the advancement of biology, research on protein methylation gradually emerged, and more and more protein methylation and its potential functions are being discovered.20
It has been reported that m6A methylation modifications are reversible and involve a combination of methylation transferases (writers), demethylases (erasers), and methylated reading proteins (readers).21,22 The “writers” include methyltransferase-like 3 (METTL3), METTL14, Wilms’ tumor 1-associated protein (WTAP), etc., whose main role is to catalyze the m6A modification of adenylate on mRNA. Demethylases [including fat mass and obesity associated (FTO) and ALKHB5] catalyze the m6A demethylation. The main function of “reader” proteins has been recognized; it binds to bases with m6A modification, thereby activating downstream regulatory pathways, such as RNA degradation and microRNA (miRNA) processing.
The development of m6A detection technology is of great significance for in-depth research on its functions in biological processes.23 Specific technologies (such as thin-layer chromatography, dot blotting, and liquid chromatography-tandem MS) have played important roles in detecting overall changes in RNA m6A levels. Furthermore, as the technology advances, more high-throughput sequencing techniques have been developed to more accurately locate and quantify m6A modification sites. Moreover, in recent years, high-throughput m6A sequencing technology has made significant progress, providing an important tool for studying the role of m6A modification in various biological processes. High-throughput sequencing helps to precisely localize m6A modification sites at the transcriptome level. Additionally, the emergence of antibody-independent sequencing techniques provides a more reliable method for validating the m6A sites. This technique avoids potential non-specific issues associated with antibodies, ensuring more accurate detection of m6A sites. Meanwhile, various new methods have been developed in recent years for the detection of single-gene m6A sites. These methods can accurately detect m6A modifications in DNA through signal amplification mechanisms without relying on antibodies or radioactive labels. These advancements not only enhance the accuracy and efficiency of m6A detection but also provide new tools and perspectives for studying the functions of m6A in various biological contexts.
The extensive and diverse nature of protein methylation makes it a hot topic in research. Through in-depth research on protein methylation, scientists have not only revealed its regulatory roles in cellular physiology and diseases but also provided a theoretical foundation for developing new therapeutic strategies. As research continues to advance, the potential functions of protein methylation will be further revealed, offering new perspectives for understanding the complexity of life processes.
Acetylation
Protein acetylation is an important PTM that has been a research hotspot since its first discovery on histones in 1964.24 Acetylation is not limited to histones but also involves many non-histone substrates, participating in the regulation of various biological processes (such as transcriptional control, DNA damage repair, and energy metabolism). In the 1990s, the first histone acetyltransferase was discovered in mammals, marking a deeper understanding of acetylation mechanisms.25 Histone acetyltransferases (HATs) not only function on histones but also modify non-histone proteins, regulating various biological processes within cells.
Acetylation can be catalyzed not only by acetyltransferases but also by non-enzymatic mechanisms. In enzymatic acetylation, the acetyl group is typically provided by acetyl-coenzyme A (acetyl-CoA) and transferred to the N-terminus or lysine residue of a protein under the action of acetyltransferase. According to the different modification positions, acetylation can be divided into two categories: acetylation at the N-terminal of the protein and acetylation on the lysine of the protein. Acetylation affects protein function by regulation of protein states (such as protein stability, enzyme activity, or subcellular localization) or by control of protein-protein and protein-DNA interactions. Based on homology to yeast histones, histone deacetylases (HDACs) in humans can be divided into four categories: I, II, III, and IV. Category I HDAC primarily includes HDAC1, HDAC2, HDAC3, and HDAC8, which play core roles in cell proliferation, differentiation, and development. Category II HDAC can be further subdivided into IIa and IIb. Among them, IIa includes HDAC4, HDAC5, HDAC7, and HDAC9, which are important in myocardial development, bone formation, adipocyte differentiation, and innate immunity.26,27 Category III HDAC, also known as Sirtuins, includes SIRT1 to SIRT7, which play significant roles in neuronal survival, proliferation, and stress response regulation.28
Acetylating assays provide a convenient method for detecting the acetylated state of proteins. These kits typically contain specific antibodies that can recognize acetylated lysines, helping researchers quickly screen and analyze acetylated proteins in samples. Additionally, acetylation affinity MS has been widely used in acetylation research. By using specific acetyllysine antibodies for protein enrichment and combining it with high-resolution MS analysis. Immunofluorescence antibody technology is also an important tool for studying acetylation, which provides crucial visual evidence for understanding the specific role of acetylation in cellular functions by using antibodies that specifically recognize acetylated lysines.
The dynamic balance between histone acetylation and deacetylation is tightly implicated in diverse conditions and diseases, including immune disorders, cell senescence, tumors, etc. Hence, acetylation plays multiple roles in the occurrence and development of many diseases. In-depth study of the mechanism of acetylation not only helps to reveal the complex biological processes in cells, but also provides new strategies and directions for disease prevention and treatment.
Ubiquitination
Ubiquitination is an important protein modification process first discovered in 1975.29 Ubiquitin (Ub) is a polypeptide composed of 76 amino acids, with high conservation and widespread expression in all eukaryotes. It has been evidenced that ubiquitination regulates various biological processes (such as protein degradation, signal transduction, and cell cycle control) by covalently attaching Ub molecules to target proteins.30 Meanwhile, the reverse process of ubiquitination, namely deubiquitination, is equally important. Deubiquitinases (DUBs) could maintain the dynamic balance of ubiquitination in cells by removing Ub molecules from proteins. These enzymes play crucial roles in various aspects of cellular behavior and exhibit selectivity for specific types or locations of Ub chains.
The ubiquitination process involves the synergistic action of Ub-activating enzyme E1, Ub-binding enzyme E2, and Ub ligase E3.31 First, the E1 enzyme activates Ub through an ATP-dependent mechanism, forming an ubiquitin-acyl-sulfinyl intermediate. This step initiates the ubiquitination cascade, ensuring that Ub can be effectively transferred to the E2 enzyme. Next, the activated Ub is transferred to the E2 enzyme via the E1 enzyme, forming an E2-Ub complex. E2 enzyme plays a crucial role in Ub transfer as it not only selects appropriate E3 enzyme but also participates in substrate modification. The structural diversity and functional richness of E2 enzymes enable them to bind to various proteins, thus playing a central role in ubiquitination signaling. Finally, E3 ligases are responsible for transferring Ub from the E2-Ub complex to target proteins. The selectivity and specificity of E3 enzymes determine the diversity of ubiquitinated substrates and the precision of signals. Taken together, these enzymes link Ub molecules to target proteins through multi-step reactions, forming polyubiquitin chains that label proteins for degradation or regulation of their function.
Currently, various technical approaches have been indicated for studying ubiquitination, which plays a crucial role in revealing the complexity of ubiquitinization systems.32 First, activity-based probes (ABPs) are important tools for studying DUBs, which can be generated as novel reagents through chemical synthesis and conjugation methods for ubiquitinization research. Second, experiments based on Ub tagging are one of the key methods for studying ubiquitination. By using engineered Ub molecules (such as K0-Ub), it is possible to effectively identify ubiquitination sites of target proteins. Additionally, ubiquitinationomics based on MS holds a significant position in ubiquitination research. Modern MS technology can not only identify and characterize hundreds of ubiquitinated substrates in a single analysis, but can also quantitatively analyze the topological structure of Ub chains. Finally, analyzing the ubiquitination sites for anti-digly antibodies is also a commonly used method, by which ubiquitinated peptides can be specifically enriched and analyzed to identify specific ubiquitination sites. All these new technologies collectively support the development of ubiquitination.
Ubiquitination is a crucial PTM of proteins, playing a pivotal role in regulating various biological processes within cells, including cell proliferation, DNA repair, replication, transcription, protein degradation, autophagy and apoptosis, innate immunity, and signal transduction. Dysfunction in ubiquitination is tightly associated with the onset of multiple diseases, such as inflammatory disorders, cancer, and neurodegenerative diseases. Hence, a deeper understanding of the mechanism of ubiquitination in cellular processes and diseases is of great significance to develop new therapeutic strategies to improve the treatment effect of related diseases.
Glycosylation
Glycosylation is one of the most complex and perhaps the most essential PTMs of the protein, first discovered in 1981.33 Glycosylation is not only prevalent in eukaryotes but has also been found in all domains of life. This modification plays a crucial role in the biosynthesis and function of proteins, affecting protein folding, intracellular localization, stability, and solubility. Glycosylation refers to a reversible enzymatic process in which sugar residues are covalently linked to proteins or lipids via glycosyltransferases or glycosidases.34 Glycosylation can occur in the cytoplasm, cell membrane, endoplasmic reticulum, and Golgi apparatus, forming complex sugar polymers. Among them, N-glycosylation and O-glycosylation are the two most common types. N-glycosylation primarily occurs in the endoplasmic reticulum, where oligosaccharide transferases (OSTs) transfer pre-assembled oligosaccharides to the asparagine residues of proteins, which is a process crucial for eukaryotic life activities. On the other hand, O-glycosylation typically occurs in the Golgi apparatus, involving multiple glycosyltransferases, mainly linking to serine and threonine residues. Additionally, O-glycosylation has unique types in plants, different from those in animals or prokaryotes; it regulates the function of secreted or nuclear proteins by modulating transcription, mediating localization, and degradation. Moreover, C-glycosylation and S-glycosylation are also important forms of glycosylation. C-glycosylation involves the covalent binding of a sugar group to a tryptophan residue, while S-glycosylation is the binding of a sugar group to a cysteine residue. These glycosylation forms have significant potential applications in the development of natural products, especially in the development of new drugs. Delving into these glycosylation mechanisms and their biological functions can help us better understand their diversity and importance within organisms.
There are diverse research methods for protein glycosylation, ranging from traditional biochemical techniques to modern high-throughput analytical methods. MS plays a crucial role in glycosylation research, especially in the field of glycopeptidomics. Specifically, MS can be used to identify glycosylation sites and measure the composition, sequence, branching, and relative abundance of sugar chains. Chemical glycoprotein spectroscopy is a method that combines chemical tools and MS techniques to identify specific glycosylation sites and glycan chain structures, thereby regulating protein function. Additionally, glycosylation analysis can also be achieved by detecting glycosyltransferase and glycosidase activities. These methods include radiochemistry, chromatography, and spectrophotometry. Lecithin analysis is another important method that helps reveal the function of glycosylation in biological processes by identifying interactions between glycoproteins and glycan-binding proteins. In short, with the continuous advancement of technology, the methods for studying protein glycosylation have become more and more diversified and accurate. The combination of these methods provides a powerful tool for understanding the role of glycosylation in biology.
Dysregulation in glycosylation affects the onset of human diseases, including tumors, diabetes, immunoinflammatory disease, etc. In clinical applications, glycosylation research has also provided new biomarkers for early disease diagnosis and patient stratification. Due to advances in analytical methods and software, glycosylation has become a key target for identifying disease biomarkers and plays a significant role in biopharmaceutical research. Therefore, the study of N-glycosylation, a complex PTM, not only helps to reveal the fundamental mechanisms of life processes but also provides new insights into disease diagnosis and treatment.
Lactylation
In 2019, a novel histone acylation code, known as lactylation, was discovered. Researchers have identified that lactylation occurs on lysine residues of histones in both human and mouse cells, which can directly stimulate gene transcription.35 This discovery highlights the intricate relationship between cellular metabolism and gene regulation, as lactate, a by-product of glycolysis, serves not only as an energy source but also as a signaling molecule that influences gene expression through histone modification.
Enzymatic lysine lactylation involves the transfer of a lactyl group from lactyl-CoA to lysine residues, a process catalyzed by the lysine acetyltransferase (KAT) enzyme P300. This modification is regulated by lactyl-CoA, which serves as the acyl donor in this reaction.36 In this context, P300 plays a crucial role as it facilitates the transfer of the lactyl group to specific lysine residues on histones, thereby influencing gene expression and cellular processes.37 The non-enzymatic lysine propionylation originates from methylglyoxal, a by-product of glycolysis, leading to the formation of lactoylglutathione (LGSH). This modification is intricately linked to cellular glucose metabolism, in which lactate plays a pivotal role. Additionally, the interplay between lactate and other metabolic substrates (such as glucose and fatty acids) highlights the complexity of metabolic regulation. Lactate can serve as an energy source for skeletal muscles and other tissues, influencing the metabolism of glucose and fatty acids. This interaction underscores the dynamic nature of metabolic pathways and their regulation by various metabolites, including lactate.
Immunochemical and MS techniques utilizing specific antibodies are pivotal for the detection of lactylation. These methods leverage the high specificity of antigen-antibody interactions, which are widely employed in bioanalytical and clinical chemistry for protein analysis. On the other hand, MS provides a powerful alternative that can offer high sensitivity and specificity in detecting and quantifying specific protein sequences. Recent advancements in MS (including high-resolution MS and tandem MS) have further improved the detection of PTMs. These techniques allow for the precise identification and quantification of lactylated peptides.
Lactylation is pivotal in gene expression, cell differentiation, and inflammatory responses. It has been increasingly recognized for its involvement in various pathological conditions, including cancer, immunoinflammatory disease, liver disease, etc. As has been confirmed previously, in the context of cancer, lactylation is intricately linked to the metabolic reprogramming of tumor cells, commonly referred to as the Warburg effect, where increased glycolysis leads to elevated lactate production.38 This lactate is not only a metabolic by-product but also acts as a signaling molecule that can modify histones, thereby influencing gene expression and promoting tumor progression. Overall, the discovery of histone lactylation adds to the growing list of histone PTMs that play crucial roles in regulating gene expression and cellular differentiation. These PTMs (including acetylation, methylation, and now lactylation) form a complex language that cells use to interpret metabolic cues and translate them into specific transcriptional outcomes. It’s essential to understand this language to decipher the epigenetic mechanisms underlying various physiological and pathological conditions.
Citrullination and Carbamylation
Citrullination is a calcium-dependent post-translational modification in which the guanidinium group of arginine is converted into a neutral urea group, forming citrulline.39 This reaction is catalyzed by peptidylarginine deiminases (PADs), primarily PAD2 and PAD4 in mammals.40 The modification results in a slight increase in molecular mass and the loss of a positive charge, thereby altering the isoelectric point, hydrogen bonding, and protein folding. These structural changes can affect protein stability, activity, and molecular interactions. The enzymatic activity of PADs requires elevated intracellular calcium levels and a reducing environment to maintain active-site thiols. In contrast, oxidative conditions and extracellular environments generally suppress PAD function. Due to these strict requirements, citrullination is thought to be a tightly regulated event, often linked to cellular stress, differentiation, or apoptosis.41 Moreover, citrullinated proteins may display altered antigenicity due to structural changes, potentially generating neoepitopes with enhanced affinity for certain MHC molecules.42,43
Carbamylation, in contrast, is a non-enzymatic and irreversible modification in which isocyanic acid reacts with lysine residues or protein N-termini to form homocitrulline.44 This process occurs spontaneously under conditions of elevated urea or inflammation, where cyanate is generated from urea decomposition or myeloperoxidase (MPO)-mediated thiocyanate oxidation.45 Smoking and chronic inflammatory states are known to promote carbamylation.46 Like citrullination, carbamylation alters the charge and structure of proteins, potentially impairing their function and clearance. Modified proteins such as carbamylated hemoglobin or LDL have been linked to pathological states including renal failure and vascular disease.47 Homocitrullinated peptides may also acquire immunogenic properties distinct from their unmodified counterparts. Detection of citrullinated and carbamylated proteins relies on mass spectrometry and specific antibody-based methods. Advances in enrichment techniques and high-resolution MS have improved sensitivity for both modifications.48
Together, citrullination and carbamylation represent charge-altering PTMs that influence protein conformation and potentially create novel antigenic determinants. Their regulation by calcium, oxidative stress, and metabolic byproducts reflects a close connection between cellular signaling and protein chemical remodeling.
PTMs and RA
PTMs are tightly implicated in the pathogenesis of RA. These modifications (including phosphorylation, methylation, acetylation, etc.) play key roles in inflammation, apoptosis, and angiogenesis, as shown in Table 1. In-depth study of the specific mechanisms of these modifications may help provide new ideas and strategies for the diagnosis and treatment of RA.
 |
Table 1 Implications of Methylation, Acetylation, and Ubiquitination in RA |
The therapeutic effects of TCM on RA may be achieved by regulating PTMs to affect RA progression. Certain TCM components can affect protein PTMs, thereby modulating immune responses and alleviating RA symptoms and progression, as indicated in Table 2. This mechanism provides new perspectives and possibilities for the application of TCM in RA treatment.
 |
Table 2 TCM Treatment on RA by Regulating PTMs |
Phosphorylation and RA
In RA, abnormal phosphorylation can lead to immune system dysregulation, thereby causing chronic inflammation and joint damage.49 Research has also shown that long non-coding RNA (lncRNA) plays a significant role in the pathogenesis of RA.50 LncRNA can regulate gene expression through various mechanisms, including affecting the function of phosphorylated proteins. Some lncRNAs may alter the phosphorylation state of specific proteins by interacting with them, thereby affecting cell proliferation, migration, and inflammatory responses. For example, it has been shown that lncRNA nuclear paraspeckle assembly transcript 1 (NEAT1) targets miR-129 and miR-204 to inhibit the activation of the MAPK signaling pathways and reduce the phosphorylation levels of ERK1/2, thereby suppressing RA fibroblast-like synoviocyte (FLS) proliferation and decreasing the incidence of synovitis.51 Another study has shown that lncRNA LOC100912373 may upregulate PDK1 expression by sponging miR-17-5p, accelerate the phosphorylation of AKT, and induce the proliferation of RA-FLS.52 Similarly, the lncRNAGAS5/miR-128-3p/HDAC4 axis has been reported to regulate RA progression through phosphorylated Akt.53 In a study by Yang et al, lncRNA H19 activates the NF-κB and JNK/p38 MAPK signaling pathways by promoting TAK1 phosphorylation, thereby enhancing the inflammatory response of RA-FLS and inhibiting apoptosis.54 This reveals the potential role of lncRNA H19 in RA and provides a theoretical basis for its potential as a therapeutic target. In recent studies, Linc00324 is found upregulated in RA, which targets miR-10a-5p to activate NF-κB phosphorylation and mediates RA inflammatory responses.55 The NF-κB signaling pathway plays a crucial role in the inflammatory response of RA, and its phosphorylation leads to the production of various pro-inflammatory cytokines (such as IL-1 and TNF-α) that play significant roles in RA pathogenesis. The above findings underscore that lncRNA plays important roles in the pathogenesis and treatment of RA by mediating protein phosphorylation and other mechanisms. An in-depth study on the function and regulatory mechanism of this process in RA will help develop new therapeutic strategies and targets, and bring more effective treatment options for RA patients.
TCM has shown significant efficacy in treating RA, in which one mechanism is achieved by inhibiting the phosphorylation of the NF-kB and JAK2/STAT3 signaling pathways. A prior study has shown that morroniside can prevent RA joint destruction by suppressing the activation of the phosphorylation of NF-κB/MMPs, thereby preventing RA-FLS invasion.56 Additionally, a study by Zhou has demonstrated that Fengshi Liuhe Decoction (FLD) can downregulate the Fzd6 protein level and inhibit the phosphorylation of key genes p-p65 and p-IκBα in the NF-κB signaling pathway, thereby reducing the inflammatory response of FLS in collagen II–induced arthritis (CIA) rats.57 Ganoderma lucidum polysaccharide peptide (GLPP) has also been highlighted for its potential therapeutic effects on RA by targeting key inflammatory pathways.58 Specifically, GLPP has been shown to decrease the phosphorylation levels of p65, IκB-α, and ERK1/2, which are critical components of the NF-κB and MAPK signaling pathways. Moreover, JAK2/STAT3 phosphorylation also plays an important role in RA pathogenesis, especially in immune inflammation. Wutou Decoction (WTD), a classic TCM formula, has shown clinical efficacy in RA treatment. It has been shown that WTD improves regulatory T cell (Treg) stability, balances CD4+ T cell subsets, and attenuates RA joint inflammation by inhibiting JAK2/STAT3 phosphorylation.59 Chrysoeriol is a flavone found in medicinal herbs, which has been commonly used in treating RA. Mechanistic studies have revealed that chrysoeriol could inhibit the activation/phosphorylation of JAK2/STAT3, thereby inducing RA-FLS apoptosis; these results provide pharmacological and chemical evidence for the traditional use of chrysoeriol-containing herbs in treating RA.60 Taken together, these findings underscore the importance of continued research into natural products as potential therapeutic agents for RA management.
Methylation and RA
It is well-known that RNA methylation is an important epigenetic modification in eukaryotic cells and plays a pivotal role in RA development and progression, as it can modulate all aspects of RNA regulation (processing, splicing, transport, and translation) and target both coding and non-coding RNAs,61 as revealed in Figure 2. Jiang et al have identified 206 differentially expressed methylated genes after performing m6A-seq analysis on MH7A cells.62 Among these, WTAP, RIPK2, JAK3, and TNFRSF10A expressions are consistent with the sequencing results, and these genes play important roles in various biological processes enriched in inflammation, cell proliferation, and apoptosis. In another study, m6A-sequencing and RNA-sequencing analysis on RA peripheral blood mononuclear cells (PBMCs) have revealed 10 genes (CD86, RAB20, XAF1, FOLR3, LTBR, KCNH8, DOK7, PDGFA, PITPNM2, and CELSR1) with concomitantly differential methylation in enhancers/promoters/gene bodies.63 Furthermore, Wan et al have revealed the changes in the expression of methylated proteins in RA synovial tissue through MeRIP-sequencing technology; further analysis has revealed that SHCBP1 and NXPH3 expressions are increased in RA patients, which are closely related to the inflammatory response and macrophage activation in RA.64 In-depth research on these genes help us better understand the role of m6A methylation in cellular physiological and pathological processes, providing new ideas and methods for RA diagnosis and treatment.
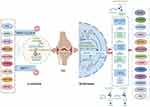 |
Figure 2 The mechanism of methylation and acetylation in RA (by figdraw). |
Accumulating studies have demonstrated that m6A “writers”, m6A “reader” proteins, and m6A “erasers” are aberrantly expressed in RA, suggesting that methylases serve critical roles in the regulation of RA pathogenesis. For example, Luo et al have manifested that the mRNA expression of ALKBH5, FTO, and YTHDF2 in RA patients is significantly decreased compared to that in controls.65 Further investigation has revealed that decreased expression of ALKBH5, FTO, and YTHDF2 in peripheral blood is a risk factor for RA. These findings provide new potential targets for the early diagnosis and treatment of RA. Additionally, the involvement of METTL3 in RA is gaining attention, primarily due to its role in promoting inflammatory responses and accelerating the progression of RA. For example, METTL3 has been found to modulate the NF-κB signaling pathway, thereby reducing lipopolysaccharide (LPS)-induced macrophage inflammatory responses and influencing RA progression.66 In the context of RA, increased expression of METTL3 has been found to be associated with synovial hyperplasia and bone destruction.67 METTL14 has also been shown to promote the progression of RA. It has been evidenced that METTL14 is associated with levels of C-reactive protein (CRP) and rheumatoid factor (RF), highlighting the potential clinically relevant discoveries.68 Specifically, METTL14 influences the polarization state of macrophages by regulating the MAPK signaling pathway, thereby exacerbating the inflammatory response in RA. WTAP, as a key component of m6A methylation, is a nuclear protein associated with the regulation of proliferation and apoptosis. Jiang et al have found that WTAP-mediated m6A modification of TRAIL-DR4 suppresses MH7A cell apoptosis, which offers a new focus and avenue for the clinical treatment of RA.69 In addition to regulating cellular pyroptosis in RA, WTAP also involves enhancing the stability of RA-FLS through m6A modification of NLRP3, thereby promoting pyroptosis and inflammatory responses.70 FTO, the first identified demethylase, has also been found to promote the progression of RA. It has been evidenced that FTO increases RA-FLS migration, invasion, and inflammatory response through m6A-IGF2BP1-dependent mechanisms.71 Moreover, FTO inhibits RA through the NSUN2/SFRP1/Wnt/β-catenin signal axis.72 Additionally, ALKBH5 and YTHDC1 also play crucial roles in RA, especially in regulating the migration, invasion, and inflammatory response of FLS.73,74 Furthermore, hypoxia-induced ALKBH5 expression promotes RA-FLS aggression and inflammation by regulating CH25H m6A stability.75 IGF2BP3 is an important RNA-binding protein that plays a crucial role in the progression of many diseases, including RA. IGF2BP3 has been shown to promote the migration and invasion of FLS in RA through the RRM2/AKT/MMP-9 pathway, thus accelerating RA progression.76 Further investigation of these mechanisms may provide new targets and strategies for the treatment of RA.
Several studies have indicated that m6A is tightly implicated in regulating ncRNA, potentially contributing to the pathogenesis of RA. For example, Wan et al have revealed that WTAP participates in the polarization process of RA macrophages by regulating the circ_0066715/miR-486-5p/ETS1 axis.77 Another research has found that WTAP regulates m6A modification of lncRNA MAPKAPK5-AS1 to promote inflammation and inhibit apoptosis.78 This process is achieved by interacting with miR-146a-3p to influence SIRT1 expression, and then regulating the NF-κB signaling pathway. Moreover, hyper-methylated hsa_circ_0007259 can bind hsa_miR-21-5p for competitive endogenous RNA (ceRNA), thereby preventing hsa_miR-21-5p from inhibiting target genes STAT3, which is implicated in the pathogenesis of RA.79 Therefore, the combination of m6A methylation and ceRNA networks not only provides new insights into the pathogenesis of RA but also offers new targets and methods for RA diagnosis and treatment. These findings suggest that targeting m6A modifications and ceRNA networks may help to develop new therapeutic strategies for RA.
Triptolide (TP) is one of the main bioactive ingredients of Tripterygium wilfordii Hook F (TWHF) in the Euonymus family, which has been proven to have therapeutic potential in the treatment of RA.80 In previous studies, TP has been identified as a target gene in RA using MeRIP-seq and RNA-seq technologies, including genes such as TUBB2A, IGF2BP3, DYNC1I1, and FOSL1.81 Identification of these genes provides a foundation for further understanding the mechanisms by which TP functions in RA. Additionally, it has been found that TP has a high binding affinity with IGF2BP3, suggesting that IGF2BP3 may be an important potential target for TP. Sarsasapogenin (Sar) is a sapogenin extracted from the TCM herb Anemarrhena asphodeloides Bung, which has anti-inflammatory benefits. As has been stated previously, Sar could modulate the biological behavior of RA-FLS by impairing m6A methylation of TGM2 mRNA and downregulating TGM2 expression.82 Sar may be a novel anti-RA drug by affecting m6A modification of TGM2. Additionally, Artemisinin (ATT), as a natural compound, has shown great potential in the treatment of RA in recent years.83 Specifically, ATT could restrain RA-FLSs migration and invasion by affecting METTL3-mediated m6A modification, especially the modification of ICAM2 mRNA, offering a new strategy for RA treatment. An in-depth study of this mechanism will not only help understand the pathophysiological mechanism of RA from the perspective of m6A methylation modification, but also provide a theoretical basis for developing TCM treatment methods.
Acetylation and RA
Histone acetylation is a reversible epigenetic modification. HDAC regulates and participates in the pathogenesis of RA from multiple perspectives. Acetylation proteomics studies have revealed 29 acetylated protein sites in RA PBMCs, with ENO1 being the most acetylated. They have also found that the acetylation of ENO1 is regulated by HDAC1, and its enzyme activity is upregulated due to acetylation.84 This suggests that high acetylation of ENO1 in RA patients may play a role in the pathophysiology of RA by increasing energy supply derived from glycolysis to maintain activated lymphocytes.
SIRT1 is an NAD+-dependent protein deacetylase and plays important roles in RA. It has been shown that 1, 25(OH)2D3 enhances p53 activity by promoting SIRT1-mediated p53 acetylation, thereby promoting the apoptosis of synovial cells and inhibiting the pathological progression of RA.85 Another study has indicated that the SIRT1/AMPKα signaling exerts anti-inflammatory activities by regulating M1/M2 polarization, thereby reducing inflammatory responses in RA.86 Otherwise, Li et al have suggested that SIRT1 expression is notably lower in RA patients’ synovial tissue and FLS compared to that in healthy controls.87 Overexpression of SIRT1 can significantly inhibit the proliferation, migration, and invasion of RA-FLS, accompanied by markedly increased apoptosis and caspase-3 and −8 activities of RA-FLS. Regarding inflammatory phenotypes, research has found that SIRT1 remarkably reduces the secretion of TNF-α, IL-6, IL-8, and IL-1β by RA-FLS. Mechanistic studies have further revealed that SIRT1 inhibits the NF-κB pathway by reducing the expression, phosphorylation, and acetylation of the p65 protein. These research findings indicate that SIRT1 plays a crucial role in the pathophysiological process of RA by enhancing the expression or function of SIRT1, and suppressing synovial hyperplasia and inflammation helps to achieve the therapeutic benefits against RA. HDACs are enzymes that play major roles in the epigenetic regulation of gene expression through the modification of histones. Increasing evidence has highlighted HDACs as active participants in RA progression. Studies have shown that HDAC1 is highly expressed in RA, and silencing HDAC1 mitigates synovial cell hyperplasia and synovial inflammation in CIA mice by elevating miR-124 and MARCKS expressions. These results suggest that combining the regulatory effects of HDACs and miRNA may help to develop more effective therapeutic strategies to control the progression of RA.88 Alternatively, a study has shown that miR-138 regulates RA-related inflammatory cytokines in RA through HDAC4/PGRN or HDAC4/NF-κB, which uncover a new molecular mechanism in RA from the perspective of acetylation modifications.89 Other studies have also discovered that HDAC5 is a novel inflammatory mediator in RA, and inflammatory cytokines epigenetically regulate RA-FLS activation by suppressing HDAC5 expression.90 Beyond that, HDAC6 is found upregulated in the synovial tissue of RA patients, and inhibiting HDAC6 can suppress the inflammatory and destructive activity of RA-FLS and reduce the severity of arthritis.91 Taken together, HDAC inhibitors could be an effective treatment for RA, which can improve patient symptoms by regulating synovial cell function and inflammation.
Exploring the role of acetylation modifications in the treatment of RA is of significant importance, especially in the context of TCM. TCM has shown promising results in the prevention and treatment of RA, and its therapeutic effects and minimal adverse reactions have been confirmed by numerous studies. The active components of TCM (such as terpenoids, flavonoids, and alkaloids) have been found to exert anti-RA effects through various mechanisms.92 For example, Wutou Decoction (WTD) is a widely used TCM for alleviating the symptoms of RA.93 Recent studies have shown that WTD may exert its anti-inflammatory effects through SIRT1-mediated mechanisms.94 Specifically, WTD potentially alleviates RA through SIRT1-mediated downregulation of HMGB1 and NF-κB acetylation. These results provide a scientific basis for the use of TCM as an effective treatment method. Additionally, baicalin, as a natural flavonoid compound, has also been proven to have significant anti-inflammatory effects on RA. Specifically, a study on SIRT1 has shown that baicalin can effectively reduce the inflammatory response in RA by downregulating the acetylation of NF-κB p65 and enhancing the deacetylation level of SIRT1. Cryptotanshinone (CTS) is an active ingredient extracted from the Chinese herb Salvia miltiorrhiza and has been studied for its therapeutic effects on RA. In the pathological process of RA, P300, a histone acetyltransferase, plays a pivotal role as it exacerbates inflammatory responses and joint damage by promoting the acetylation of STAT3, thereby enhancing its transcriptional activity. It has been shown that CTS can downregulate p300-mediated STAT3 acetylation, thus showing therapeutic potential for RA.95 Furthermore, Seong et al have found that Delphinidin, a specific inhibitor of histone acetyltransferase, can prevent inflammatory signaling by inhibiting the acetylation of NF-κB in MH7A cells.96 These findings further highlight the importance of histone acetylation in RA inflammatory responses. Delving deeper into these mechanisms may help develop more effective anti-arthritic treatments.
Ubiquitination and RA
Ubiquitination is an important biological modification that regulates protein function, stability, and localization through the covalent attachment of Ub molecules. It plays a crucial role in various biological processes, including cell cycle regulation, signal transduction, DNA repair, and immune responses. In the pathogenesis of RA, ubiquitination also plays a pivotal role.97 First, the role of ubiquitination in RA can be demonstrated by the regulation of T cells and inflammatory responses. It has been shown that E3 Ub ligases STUB1 may induce the imbalance of Th17/Treg cells by promoting ubiquitination of AHR via K63 chains, which could serve as a potential therapeutic target for RA.98 Alternatively, another study has revealed that STUB1 can promote the differentiation of T-cell differentiation in RA by mediating the activation of the mTORC1 pathway through ubiquitination of p62.99 Furthermore, E3 Ub ligases play a crucial role in the pathogenesis of RA by regulating cell death pathways and promoting abnormal proliferation of FLS. Specifically, BIRC3, as an E3 Ub ligase gene, can promote the secretion of inflammatory cytokines and FLS proliferation, thereby influencing RA progression.100 The Ub-specific protease 2 (USP2) is known to have a substantial influence on the regulation of several cellular processes. Liu et al have shown that USP2 interacts with TRAF2 and promotes RA-FLS proliferation and inflammation by facilitating the removal of ubiquitination chains from TRAF2.101 The Mid1 protein, as an E3 Ub ligase, plays a crucial role in various biological processes, especially in inflammatory diseases. In RA, the ubiquitination of DPP4 by Mid1 is considered a key factor in promoting the proliferation and invasion of RA-FLS.102 This mechanism reveals a new pathway for synovial activation and identifies Mid1 as a promising target for RA treatment interventions. Beclin1 is a crucial regulator of autophagy, and its ubiquitination state directly affects the activity of autophagy. K63 ubiquitination of Beclin1 is one of the key steps in initiating autophagy. Additionally, Bcl-2 is an anti-apoptotic protein known to inhibit autophagy when it binds to Beclin1. As has been evidenced previously, synovial water channel protein 1 (AQP1) in RA-FLS enhances the binding of Bcl-2 to Beclin1 by reducing Beclin1 K63 ubiquitination, thereby inhibiting autophagy.103 This finding not only deepens our understanding of the regulation mechanism of autophagy, but also provides a potential target for RA treatment.
LncRNA NEAT1 is frequently dysregulated in RA, and its mediated downstream protein ubiquitination is one of the possible molecular mechanisms of RA development. It has been demonstrated that lncRNA NEAT1 competitively binds to miR-23a, affecting the ubiquitination process of MDM2 and leading to the degradation of SIRT6, which in turn promotes RA-FLS inflammation and proliferation.104 Similarly, STAT3 is a downstream molecule for lncRNA NEAT1, and its cellular level can be positively targeted and regulated by lncRNA NEAT1 via reducing the ubiquitination level. This suggests that knockdown of lncRNA NEAT1 positively relieves arthritis degree in CIA mice through reducing the ubiquitination level of STAT3.105 This regulatory mechanism could not only offer new insights into understanding the role of lncRNA NEAT1 from the perspective of ubiquitination modification, but also offer potential targets for RA treatment.
In recent years, significant progress has been made in the study of RA treatment from the perspective of ubiquitination. β-Indole-3-acetic acid (IAA), as an endogenous ligand, exerts effects through the aryl hydrocarbon receptor (AhR) pathway. A previous study has shown that IAA can reduce the ubiquitination of Foxp3, a Treg transcription factor, via the AhR-TAZ-Tip60 pathway, thereby enhancing Treg cell function and alleviating CIA.106 This finding offers new insights into the treatment of RA, suggesting that IAA may alleviate RA symptoms by regulating the ubiquitination of Foxp3. UBA1 is the initial enzyme in the ubiquitination cascade reaction, and its activity is crucial for the transfer of Ub molecules to target proteins. It has been demonstrated that Auranofin could target UBA1 and enhance the activity of UBA1 by promoting the Ub transferase process, thereby playing a role in the treatment of RA.107 This mechanism involves the activation and transfer processes of Ub, especially the critical interaction between E1, E2, and E3 enzymes. Menthone, as a monoterpene compound, exhibits significant anti-inflammatory properties. Studies have found that mint ketone can inhibit the activation of the type I interferon (IFN-I) signaling pathway by promoting the polyubiquitination of Tyk2 at its K48 residue, thereby exerting anti-inflammatory effects in arthritis models.108 Specifically, the topical application of menthol can significantly reduce local inflammation in CIA mice. In conclusion, ubiquitination plays multiple roles in the pathogenesis of RA, providing new possibilities for the treatment of RA by regulating immune response and signaling pathways. Further exploration of the specific mechanism of ubiquitination in RA may help to develop more effective treatments.
Glycosylation and RA
Glycolysis plays a significant role in RA, and enhanced glycolysis is closely associated with the onset of RA.109 Key glycolytic enzymes [such as hexokinase 2 (HK2), 6-phosphofructo-2-kinase 3 (PFKFB3), and pyruvate kinase M2 (PKM2)] significantly contribute to RA development.110 Increasing the translation or transcription of glycolytic enzymes helps to directly activate RA macrophage polarization, as indicated in Figure 3. FLS are the most abundant resident cells in the synovial membrane and play critical roles in the pathogenesis of RA.111 Increased activity of these glycolytic enzymes may promote the proliferation of synovial cells and inflammatory responses, thereby exacerbating disease progression.112 RA-FLS exhibits abnormal glycolytic metabolic characteristics. This metabolic reprogramming can lead to the transformation of synovial cells from a quiescent state to a highly active one, further exacerbating joint inflammation and destruction. β-1, 4-galactosyltransferase 1 (β4GalT1) is a key glycosyltransferase that regulates the invasive behavior of RA-FLS. Specifically, β4GalT1 affects the invasion of FLS by modifying the N-linked glycosylation of chemokine receptor CXCR3.113 This glycosylation modification may alter the conformation of CXCR3 or its binding ability to ligands, thereby regulating the migration and invasion of FLS. Eukaryotic elongation factor-2 kinase (eEF2K) is a negative regulator of protein synthesis, which is increased in RA-FLS. eEF2K knockdown could significantly reduce inflammation, migration, invasion, glucose uptake, and lactate production of RA-FLS, indicating that targeting eEF2K may be a new strategy for RA therapy.114 Additionally, studies have found that lncRNA TUG1 regulates the expression of miR-34a-5p and LDHA, influencing the glucose metabolism and apoptosis processes in RA-FLS, suggesting that targeting lncRNA TUG1 could be an effective therapeutic approach to inhibit glycolysis of FLS for RA treatment.115
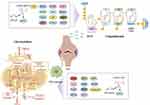 |
Figure 3 The mechanism of glycosylation and ubiquitination in RA (by figdraw). |
In the pathological process of RA, the enhancement of glycolysis not only affects synovial cells but may also impact the function of other immune cells. For example, macrophages in RA patients also show altered transcriptional regulation of the glycolytic pathway, which may drive macrophages to produce pro‐inflammatory responses. It has been shown that cytoplasmic zinc induces glycolytic processes through the mTORC1 signaling pathways, significantly promoting the production of IL-1β, a key pro-inflammatory cytokine.116 As a novel endogenous factor, IL-34 promotes the differentiation and survival of monocytes and macrophages by binding to M-CSFR and syndecan 1.117 Additionally, IL-34 can reprogram the metabolic state of macrophages by influencing glycolytic pathways, thereby affecting their functional performance to advance inflammatory bone destruction in RA. This mechanism provides new potential targets for the treatment of RA, especially in regulating macrophage metabolism and function. Moreover, AMPK (adenylate cyclase) plays a crucial role in energy metabolism, especially in glycolysis. In the study of RA, the lack of AMPK/SIRT1 could exacerbate inflammatory responses by promoting glycolytic-mediated monocyte inflammatory polarization. This mechanism may be related to AMPK’s role in regulating glycolysis and inflammatory polarization, further highlighting the importance of AMPK as a potential therapeutic target in RA.118 In another study, glycolysis inhibitor 2-deoxyglucose (2-DG) reduces the production of pro-inflammatory cytokines and promotes the polarization of anti-inflammatory macrophages by activating AMPK via phosphorylation and reducing NF-κB activation in adjuvant-induced arthritis rats. These findings indicate that the anti-arthritic 2-DG effect is mediated by the modulation of macrophage polarization in an AMPK-dependent manner.119 Besides, Xu et al have shown that PKM2 is highly expressed in the spleen and synovial tissues of arthritic rats, especially in ED1-positive macrophages, which promotes macrophage activation through the STAT1 signaling.120 Hence, selective metabolic targeting of PKM2 may offer new therapeutic strategies for RA. Recent studies have revealed a connection between glycolysis and inflammatory response in RA macrophages and FLS. For example, it has been found that blockade of IRAK4 and its interconnected intermediates can rebalance the metabolic malfunction by obstructing glycolytic and inflammatory phenotypes in RA macrophages and FLS.121 Therefore, regulating IRAK4 and its related signaling pathways holds significant potential in the treatment of RA. For instance, IL-27 enhances peripheral B cell glycolysis of RA patients by activating the mTOR signaling. The transcriptional regulation of glycolysis in circulating CD8+ T cells of RA patients is a critical factor for RA pathogenesis.122 Alterations in these metabolic pathways can promote the proliferation and tissue invasiveness of RA T cells, making them potential targets for therapeutic intervention. It has been shown that CD8+ effector memory cells (Tem) and CD8+CD45RA+ effector memory cells (Temra) exhibit significant changes in the transcriptional regulation of glycolysis in untreated RA patients.123 Key glycolytic enzymes (such as PFKFB3 and GAPDH) are differentially regulated, suggesting that targeting these pathways may be beneficial for RA management.
TCM has been recognized for its multifaceted approach in regulating glycolysis, which plays a significant role in the treatment of RA. For example, total saponins of anemarrhena (TSA) is a class of steroid saponins, and it’s treatment on RA mainly through downregulating the activity of PKM2 tetramer and phosphorylation of PKM2 to inhibit pathological glycolysis and induce apoptosis, thereby inhibiting the proliferation and invasion of RA FLS.124 Another study has found that TP inhibits Th17 cell differentiation through suppressing PKM2-mediated glycolysis, thereby attenuating joint inflammation of CIA mice.125 This suggests that targeting PKM2 can effectively reduce Th17 cell-mediated inflammation and provide therapeutic benefits in RA. Additionally, inhibiting macrophage polarization through the glycolytic pathway is also an important target for RA treatment. The active compound, icariin (ICA), derived from the herb Epimedium, exhibits potent anti-inflammatory properties. ICE has been shown to suppress RA by promoting an M1-to-M2 switch and suppressing glycolysis.126 Cepharanthine (CEP) is an attractive candidate for therapeutic intervention in inflammatory diseases; its inhibitory effects on macrophage polarization may be attributed to the blockage of TLRs-MyD88/IRAK4-IRF5 signaling pathway, along with suppression of overactivated glycolytic metabolism in M1-polarizing macrophages, thus ameliorating synovial inflammation and joint destruction of CIA mice.127 Berberine (BBR), as an inhibitor of inflammation, exerts key therapeutic effects on RA. Studies have confirmed that activation of AMPK is required for the BBR-mediated anti-arthritis effect by downregulating mTORC1/HIF-1α and inhibiting the glycolysis in M1 macrophages. Angiogenesis plays a crucial role in the pathogenesis of RA, and studying this process from the perspective of glycolysis may be an innovative approach.128 Additionally, α-Mangostin can reduce HIF-1α-mediated angiogenesis in adjuvant-induced arthritis rats by inhibiting aerobic glycolysis, providing a new therapeutic target for RA.129 Taken together, these findings suggest that drugs have the potential to inhibit angiogenesis and anti-inflammatory effects under glycolytic conditions, which is of great significance as it further highlights the role of drugs in metabolic regulation. These findings not only provide new perspectives for RA treatments but also offer a theoretical basis for developing novel multi-target drugs.
Lactylation and RA
Lactic acid (LA), an essential glycolytic metabolite and energy source in the body, has attracted attention regarding its role in RA. LA is not only a byproduct of cellular metabolism but can also act as a signaling molecule to regulate cell function and inflammatory responses. LA acts as an inflammatory amplifier in RA. Recent studies have further found that novel PTM lactylation mediated by LA may also play a key role in RA.130 For example, through single-cell sequencing data combined with bioinformatics and machine learning techniques, it has been found that RA plasma cells displaying the highest lactylation scores, NDUFB3, NGLY1, and SLC25A4 genes are highly expressed in RA patients, which is further verified by RT-qPCR and are positively correlated with immune cell infiltration.131 High expression of these genes may be closely related to the pathophysiological process of RA. These results highlight the critical role of lactylation in plasma cells for RA pathogenesis and identify potential biomarkers and therapeutic targets, offering novel insights into developing future therapeutic strategies. RBM25 is an important RNA-binding protein that plays a crucial role in regulating gene expression and cellular metabolism. It has been shown that decreased expression of RBM25 is identified as a major pathogenic factor in RA patients and experimental arthritis mice.132 ACLY’s RNA splicing changes are closely associated with various diseases, especially the lactylation modification of ACLY, which is considered one of the key mechanisms regulating its function. RBM25 deficiency overwhelmingly leads to ACLY protein lactylation on lysine 918/995, resulting in pro-inflammatory mediator production in macrophages of autoimmune arthritis.133 The lactylation modification of PKM2 is one of the key mechanisms regulating its function. Lactylation can inhibit the transformation of PKM2 from a tetramer to a dimer, thereby enhancing its enzymatic activity and reducing its distribution in the nucleus. It has been shown that artemisinin (ART) regulates the cell cycle through PKM2 lactylation, and directing interventions towards PKM2 in RA-FLS offers a hopeful avenue for pharmaceutical treatments of RA.132
Therefore, lactate modification plays an important role in the occurrence and development of RA. An in-depth study on the specific mechanism and function of lactate modification in RA may provide new ideas and targets for the diagnosis and treatment of RA.
Citrullination/Carbamylation and RA
Citrullination, catalyzed by calcium-dependent PADs, is a PTM that converts arginine into citrulline, resulting in altered protein charge, structure, and function. In RA, excessive citrullination breaks immune tolerance by generating neoantigens that are recognized by anti-citrullinated protein antibodies (ACPAs), a highly specific serological marker of RA.134 Citrullinated peptides show preferential binding to HLA-DRB1 alleles with the shared epitope, promoting T cell activation and autoantibody production.135 Recent studies have revealed that monoclonal ACPAs can be functionally diverse—some clones even exhibit protective effects by inducing IL-10 secretion from macrophages via FcγR2B signaling in response to citrullinated α-enolase complexes, thereby reducing osteoclastogenesis and joint damage.136 Beyond host enzymes, microbial PADs (eg, PPAD from Porphyromonas gingivalis) also contribute to citrullination and may trigger autoimmunity. Dysbiosis of oral and gut microbiota—especially the expansion of Porphyromonas gingivalis and Prevotella copri—has been implicated in RA onset.137 Another study has identified Prevotella copri as a bacterium that may contribute to the pathogenesis of the autoimmune disease psoriasis.138 These bacteria can either produce citrullinated bacterial proteins or induce citrullination of host proteins, both of which may provoke ACPA responses.
Carbamylation, in contrast, is a non-enzymatic PTM wherein cyanate (derived from urea breakdown or MPO-mediated thiocyanate oxidation) reacts with lysine residues to form homocitrulline. Anti-carbamylated protein (anti-CarP) antibodies are detectable in up to 50% of RA patients and are associated with disease onset and severity.139,140 Recent work has demonstrated that neutrophils in the RA synovial fluid (SF) are highly activated, releasing MPO and reactive oxygen species (ROS), which strongly correlate with elevated levels of carbamylated proteins in RA joints.141 Moreover, carbamylated neutrophil extracellular traps (cNETs) have been shown to promote osteoclastogenesis via TLR4 signaling, thereby contributing to bone erosion, a hallmark of progressive RA.142 Together, citrullination and carbamylation represent two pivotal PTMs that link metabolic and inflammatory stress with autoantigen generation and immune dysregulation in RA. They not only serve as biomarkers but also actively participate in shaping the disease course by modulating antigen presentation, immune complex formation, cytokine production, and tissue destruction, AS shown in Table 3. Understanding their precise roles may provide therapeutic avenues for targeted intervention in RA.
 |
Table 3 Comparative Overview of Lactylation, Citrullination, and Carbamylation in Rheumatoid Arthritis |
Conclusions
In recent years, MS techniques have identified various novel PTMs—such as glycosylation, ubiquitination, acetylation, lactylation, citrullination and carbamylation—that play vital roles in disease processes, including RA. PTMs are increasingly recognized as biomarkers for RA diagnosis, prognosis, and therapeutic monitoring. By modulating key cellular pathways, PTMs not only reflect disease activity but also offer promising targets for intervention. Targeting PTM-related enzymes may help restore immune balance and provide new avenues for RA treatment.
Research on PTMs in RA has advanced significantly with the development of MS. However, current MS-based techniques still face limitations in sensitivity, throughput, and data interpretation, which restrict the comprehensive identification and characterization of PTMs in complex disease contexts. Despite increasing evidence linking PTMs to immune regulation and inflammation in RA, their specific molecular mechanisms remain incompletely understood. Most studies remain descriptive, focusing on correlations rather than causation, and lack systematic functional validation. Addressing this gap requires robust experimental models to elucidate how PTMs influence key signaling pathways and immune cell behavior in RA pathogenesis. Given the heterogeneity of RA, individual variations in PTM patterns may contribute to diverse clinical outcomes. Integrating multiomics data—including genomics, transcriptomics, proteomics, and epigenomics—offers a promising strategy to map PTM landscapes across patient subtypes. Such approaches may lead to the discovery of new biomarkers and the development of personalized therapeutic strategies targeting disease-specific PTM signatures.
Additionally, the cross-regulation of PTMs (crosstalk) has become a research hotspot. The interactions between different PTMs can form complex PTM codes, which can be recognized by specific effectors to initiate or inhibit downstream events. This cross-regulation mechanism offers a new perspective for understanding the dynamic regulation of protein function and may reveal new therapeutic targets for RA. An intriguing aspect of exosome biology is their ability to carry proteins that have undergone PTMs, which are chemical modifications that occur after protein synthesis and can significantly alter protein function and interactions. The interaction between ncRNAs and PTMs within exosomes is a burgeoning area of research. ncRNAs (including miRNAs and lncRNAs) can regulate gene expression and are involved in various cellular processes, including PTMs-related processes. Exosomes can serve as vehicles for ncRNAs, facilitating their transport and delivery to target cells, where they can modulate PTM-related pathways. For instance, exosomes-carried ncRNAs have been shown to influence the sorting of proteins into exosomes and the regulation of PTMs, thereby affecting cellular communication and function.
Moreover, merging evidence highlights the central role of PTMs in linking autophagy, extracellular vesicle (EV) biology, and immune dysregulation in RA. Stress signals such as smoking, joint injury, or infection may induce autophagy, which in turn facilitates the generation of neoantigens through PTMs like citrullination and carbamylation—thereby contributing to the breakdown of immune tolerance. Moreover, autophagy-related machinery intersects with secretory pathways, promoting the release of PTM-modified proteins via EVs, particularly exosomes and microvesicles.143 These vesicles, mainly derived from synoviocytes and leukocytes in RA, are enriched in citrullinated proteins and inflammatory mediators, playing critical roles in antigen presentation and intercellular communication. Additionally, key autophagy regulators themselves are subject to PTMs such as ubiquitination, phosphorylation, and acetylation, which fine-tune autophagic flux and influence disease progression. Thus, the interplay between PTMs, autophagy, and EVs forms a complex regulatory axis that may underlie RA pathogenesis and offers novel targets for precision therapy.
In summary, PTMs play a crucial role in regulating the stability and function of target proteins, which in turn influences protein-protein interactions. These interactions are fundamental to almost all biological processes, providing novel and diverse mechanisms for the regulation of systems biology. Moreover, the focus on PTMs and precision medicine in drug development represents a significant shift towards more personalized and effective treatment strategies for RA. By leveraging these advanced methodologies, researchers can potentially improve therapeutic outcomes and enhance the quality of life of patients with these chronic conditions.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author.
Acknowledgments
This work was supported by the Clinical Research Project of Anhui University of Traditional Chinese Medicine in 2024 (2024YFYLCZX09), Anhui University of Chinese Medicine 2024 Annual University-Level Exploratory Research Project (AHUCM2024TS099) and National Key Discipline of Traditional Chinese Medicine - Traditional Chinese Medicine Bi Disease ([2023] No. 85).
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Seema D, Sharma S, Leung H, Sebastien V. Genetics of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2024;38(4):101968. doi:10.1016/j.berh.2024.101968
2. Alexander V, Blagov A, Grechko V, et al. Effects of Metabolic Disorders in Immune Cells and Synoviocytes on the Development of Rheumatoid Arthritis. Metabolites. 2022;12(7):1.
3. Jianting W, Jian L, Lei W, et al. The effect of long-term traditional Chinese medicine treatment on extra-articular lesions of rheumatoid arthritis patients based on propensity score matching: a retrospective cohort study. Heliyon. 2024;10(1):e23147. doi:10.1016/j.heliyon.2023.e23147
4. Shahad A-B, Gabriel ZS, Diego Z, et al. Interstitial Lung Disease in Rheumatoid Arthritis: a Review. Cureus. 2024;16(2):e53632. doi:10.7759/cureus.53632
5. Annabelle R, Machin O, Babatunde R, et al. The association between anxiety and disease activity and quality of life in rheumatoid arthritis: a systematic review and meta-analysis. Clin Rheumatol. 2020;39(5):1471–1482. doi:10.1007/s10067-019-04900-y
6. Yayun X, Wenqiang L, Lijie R. Emerging roles and mechanism of m6A methylation in rheumatoid arthritis. Biomed Pharmacother. 2024;170:116066. doi:10.1016/j.biopha.2023.116066
7. Jianting W, Jian L, Lei W, et al. Long noncoding RNA/circular RNA regulates competitive endogenous RNA networks in rheumatoid arthritis: molecular mechanisms and traditional Chinese medicine therapeutic significances. Ann Med. 2023;55(1):973–989. doi:10.1080/07853890.2023.2172605
8. Menglin Z, Qian D, Zhongxiao L, et al. New Targets and Strategies for Rheumatoid Arthritis: from Signal Transduction to Epigenetic Aspect. Biomolecules. 2023;13(5). doi:10.3390/biom13050766.
9. Min-Seon H, Jingyeong P, Yunha H, et al. Roles of Protein Post-Translational Modifications During Adipocyte Senescence. Int J Biol Sci. 2023;19(16):5245–5256. doi:10.7150/ijbs.86404
10. Shahin R, Javad Z. Posttranslational modifications in proteins: resources, tools and prediction methods. Database. 2021;2021:1.
11. Vasudevan V, Prachi A, Sagarika B. Post Translational Modification and Its Pathologic Association in Rheumatoid Arthritis: a Brief Perspective. Curr Protein Pept Sci. 2021;22(7):548–558. doi:10.2174/1389203722666210215152433
12. Shu W, Osgood Arianna O, Abhishek C. Uncovering post-translational modification-associated protein-protein interactions. Curr Opin Struct Biol. 2022;74:102352. doi:10.1016/j.sbi.2022.102352
13. Weiwei L, Sheng L, Lei N, et al. Functional interaction between long non-coding RNA and microRNA in rheumatoid arthritis. J Clin Lab Anal. 2020;34(12):e23489. doi:10.1002/jcla.23489
14. Mario L, Entwisle Samuel W, Judit V. Decoding Post-Translational Modification Crosstalk With Proteomics. Mol Cell Proteomics. 2021;20:100129. doi:10.1016/j.mcpro.2021.100129
15. Larsen Martin R, Trelle Morten B, Thingholm Tine E, et al. Analysis of posttranslational modifications of proteins by tandem mass spectrometry. Biotechniques. 2006;40(6):790–798. doi:10.2144/000112201
16. Levene PA, Alsberg CL. The cleavage products of Vitellin. J Biol Chem. 1906;2(1):127–133. doi:10.1016/S0021-9258(17)46054-6
17. Wen X, Huang Y. Regulation of Inflammatory Cell Death by Phosphorylation. Front Immunol. 2022;13:851169. doi:10.3389/fimmu.2022.851169
18. Hieu N, Kettenbach Arminja N. Substrate and phosphorylation site selection by phosphoprotein phosphatases. Trends Biochem Sci. 2023;48(8):713–725. doi:10.1016/j.tibs.2023.04.004
19. Schoenheimer R, Ratner S, Rittenberg D. Studies in protein metabolism: VII. The Metabolism of Tyrosine J. Biol Chem. 1939;127(1):333–344. doi:10.1016/S0021-9258(18)73846-5
20. Jernej M, Yang S. The winding path of protein methylation research: milestones and new frontiers. Nat Rev Mol Cell Biol. 2017;18(8):517–527. doi:10.1038/nrm.2017.35
21. Wenxiu R, Xiaoyan Z, Binglin Y, et al. Insight into m6A methylation from occurrence to functions. Open Biol. 2020;10(9):200091. doi:10.1098/rsob.200091
22. Elsabbagh Raghda A, Mona R, Carsten W, et al. Impact of N6-methyladenosine (mA) modification on immunity. Cell Commun Signal. 2022;20(1):140. doi:10.1186/s12964-022-00939-8
23. Huiping S, Feiyu Y, Zhuo Z, et al. A review of advances in analytical strategies for RNA methylation. Anal Chim Acta. 2025;1333:343154. doi:10.1016/j.aca.2024.343154
24. Allfrey VG, Faulkner R, Mirsky AE. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis J. Proc Natl Acad Sci USA. 1964;51(5):786794. doi:10.1073/pnas.51.5.786
25. Eric V, Melanie O. 50 years of protein acetylation: from gene regulation to epigenetics, metabolism and beyond. Nat Rev Mol Cell Biol. 2015;16(4):258–264. doi:10.1038/nrm3931
26. Takeo N, Weinert Brian T, Chunaram C. Functions and mechanisms of non-histone protein acetylation. Nat Rev Mol Cell Biol. 2019;20(3):156–174. doi:10.1038/s41580-018-0081-3
27. Can X, Tao Y, Mingshan L, et al. Protein acetylation and deacetylation: an important regulatory modification in gene transcription (Review). Exp Ther Med. 2020;20(4):2923–2940. doi:10.3892/etm.2020.9073
28. Shubhra P, Sheikh R. Molecular and cellular regulatory roles of sirtuin protein. Crit Rev Food Sci Nutr. 2023;63(29):9895–9913. doi:10.1080/10408398.2022.2070722
29. Goldstein G, Scheid M, Hammerling U, Schlesinger DH, Niall HD, Boyse EA. Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells J. Proc Natl Acad Sci. 1975;72(1):11–15. doi:10.1073/pnas.72.1.11
30. Yihui W, Yifei C, Xianyan T, et al. Ubiquitination regulates autophagy in cancer: simple modifications, promising targets. J Transl Med. 2024;22(1):985. doi:10.1186/s12967-024-05565-1
31. Gizem C, Hale K, Ebru O, et al. The Effect of Dysfunctional Ubiquitin Enzymes in the Pathogenesis of Most Common Diseases. Int J Mol Sci. 2020;21:1.
32. De Silva Anthony Ruvindi I, Page Richard C. Ubiquitination detection techniques. Exp Biol Med (Maywood). 2023;248(15):1333–1346. doi:10.1177/15353702231191186
33. Bause E, Legler G. The role of the hydroxy amino acid in the triplet sequence Asn-Xaa-Thr(Ser) for the N -glycosylation step during glycoprotein biosynthesis. Biochem J. 1985;195(3):639–644. doi:10.1042/bj1950639
34. Jennifer M, Elliott David J. Hallmarks of glycosylation in cancer. Oncotarget. 2016;7(23):35478–35489. doi:10.18632/oncotarget.8155
35. Zhang D, Zhanyun T, Huang H, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574(7779):575–580. doi:10.1038/s41586-019-1678-1
36. Fengyang J, Jianyun Z, Heyu Z, et al. Unlocking the multifaceted molecular functions and diverse disease implications of lactylation. Biol Rev Camb Philos Soc. 2024;100(1):172–189. doi:10.1111/brv.13135
37. Yue H, Zhenglin H, Zongjun L, et al. Lactylation: the novel histone modification influence on gene expression, protein function, and disease. Clin Epigenetics. 2024;16(1):72. doi:10.1186/s13148-024-01682-2
38. Jie C, Ziyue H, Chen Y, et al. Lactate and lactylation in cancer. Signal Transduct Target Ther. 2025;10(1):38. doi:10.1038/s41392-024-02082-x
39. Valesini G, Gerardi MC, Iannuccelli C, et al. Citrullination and autoimmunity. Mosaic of Autoimmunity. 2019;2019:117–126.
40. György B, Tóth E, Tarcsa E, et al. Citrullination: a posttranslational modification in health and disease. The International Journal of Biochemistry & Cell Biology. 2006;38(10):1662–1677. doi:10.1016/j.biocel.2006.03.008
41. Pruijn GJM. Citrullination and carbamylation in the pathophysiology of rheumatoid arthritis. Frontiers in Immunology. 2015;6:192. doi:10.3389/fimmu.2015.00192
42. Brentville VA, Metheringham RL, Gunn B, et al. Citrullinated vimentin presented on MHC-II in tumor cells is a target for CD4+ T-cell–mediated antitumor immunity. Cancer Research. 2016;76(3):548–560. doi:10.1158/0008-5472.CAN-15-1085
43. Curran AM, Girgis AA, Jang Y, et al. Citrullination modulates antigen processing and presentation by revealing cryptic epitopes in rheumatoid arthritis. Nature Communications. 2023;14(1):1061. doi:10.1038/s41467-023-36620-y
44. Long J, Parada XV, Kalim S. Protein carbamylation in chronic kidney disease and dialysis. Advances in Clinical Chemistry. 2018;87:37–67.
45. Kwon EJ, Ju JH. Impact of posttranslational modification in pathogenesis of rheumatoid arthritis: focusing on citrullination, carbamylation, and acetylation. International Journal of Molecular Sciences. 2021;22(19):10576. doi:10.3390/ijms221910576
46. Ospelt C, Bang H, Feist E, et al. Carbamylation of vimentin is inducible by smoking and represents an independent autoantigen in rheumatoid arthritis. Annals of the Rheumatic Diseases. 2017;76(7):1176–1183. doi:10.1136/annrheumdis-2016-210059
47. Hensen SMM, Pruijn GJM. Methods for the detection of peptidylarginine deiminase (PAD) activity and protein citrullination. Molecular & Cellular Proteomics. 2014;13(2):388–396. doi:10.1074/mcp.R113.033746
48. Slade DJ, Subramanian V, Fuhrmann J, et al. Chemical and biological methods to detect post‐translational modifications of arginine. Biopolymers. 2014;101(2):133–143. doi:10.1002/bip.22256
49. Cankut Ç, Rachel L, Pedro C, et al. Phosphoproteomic profiling of early rheumatoid arthritis synovium reveals active signalling pathways and differentiates inflammatory pathotypes. Arthritis Res Ther. 2024;26(1):120. doi:10.1186/s13075-024-03351-4
50. Wentao H, Xue L, Chen H, et al. LncRNAs and Rheumatoid Arthritis: from Identifying Mechanisms to Clinical Investigation. Front Immunol. 2022;12:807738. doi:10.3389/fimmu.2021.807738
51. Jie C, Xiao L, Mao L, et al. Silencing long non-coding RNA NEAT1 attenuates rheumatoid arthritis via the MAPK/ERK signalling pathway by downregulating microRNA-129 and microRNA-204. RNA Biol. 2020;18(5):657–668.
52. Chang F, Xiaoya C, Sen C, et al. LncRNA LOC100912373 modulates PDK1 expression by sponging miR-17-5p to promote the proliferation of fibroblast-like synoviocytes in rheumatoid arthritis. Am J Transl Res. 2021;12(12):7709–7723.
53. Tao P, Dehui J, Yankai J. Long non-coding RNA GAS5 suppresses rheumatoid arthritis progression via miR-128-3p/HDAC4 axis. Mol Cell Biochem. 2021;476(6):2491–2501. doi:10.1007/s11010-021-04098-1
54. Jialiang Y, Yixuan L, Lili W, et al. LncRNA H19 aggravates TNF -α-induced inflammatory injury via TAK1 pathway in MH7A cells. Biofactors. 2020;46(5):813–820. doi:10.1002/biof.1659
55. Binbin X, Faquan L, Wei B, et al. Long noncoding RNA00324 is involved in the inflammation of rheumatoid arthritis by targeting miR-10a-5p via the NF-κB pathway. Immun Inflamm Dis. 2023;11(6):e906. doi:10.1002/iid3.906
56. Yan W, Ruili Y, Xin L, et al. Morroniside Attenuates Rheumatoid Arthritis by Inhibiting Rheumatoid Synoviocytes Invasion through the NF-κB/MMPs Pathway. Ann Clin Lab Sci. 2024;54(5):604–617.
57. Fulin Z, Peilin Z, Xian J, et al. Fengshi Liuhe Decoction treatment for rheumatoid arthritis via the Fzd6/NF-κB signaling axis. Gene. 2024;920:148538. doi:10.1016/j.gene.2024.148538
58. Meng M, Lianfu W, Yang Y, et al. Ganoderma lucidum polysaccharide peptide (GLPP) attenuates rheumatic arthritis in rats through inactivating NF-κB and MAPK signaling pathways. Phytomedicine. 2023;119:155010. doi:10.1016/j.phymed.2023.155010
59. Liang H, Jiahui Y, Tingting L, et al. Wutou decoction alleviates arthritis inflammation in CIA mice by regulating Treg cell stability and Treg/Th17 balance via the JAK2/STAT3 pathway. J Ethnopharmacol. 2024;334:118463. doi:10.1016/j.jep.2024.118463
60. Jia-Ying W, Ying-Jie C, Xiu-Qiong F, et al. Chrysoeriol suppresses hyperproliferation of rheumatoid arthritis fibroblast-like synoviocytes and inhibits JAK2/STAT3 signaling. BMC Complement Med Ther. 2022;22(1):73. doi:10.1186/s12906-022-03553-w
61. Yurong H, Qiuyun X, Jun C, et al. M6A methylation modification in autoimmune diseases, a promising treatment strategy based on epigenetics. Arthritis Res Ther. 2023;25(1):189. doi:10.1186/s13075-023-03149-w
62. Hui J, Kefeng C, Chang F, et al. Transcriptome-Wide High-Throughput m6A Sequencing of Differential m6A Methylation Patterns in the Human Rheumatoid Arthritis Fibroblast-Like Synoviocytes Cell Line MH7A. J Inflamm Res. 2021;14:575–586. doi:10.2147/JIR.S296006
63. Chia-Chun T, Yuan-Zhao L, Lin C-H, et al. Next-Generation Sequencing Profiles of the Methylome and Transcriptome in Peripheral Blood Mononuclear Cells of Rheumatoid Arthritis. J Clin Med. 2019;8(9):1.
64. Lei W, Jian L, Chuanbing H, et al. Comprehensive Analysis and Functional Characteristics of Differential Expression of N6-Methyladenosine Methylation Modification in the Whole Transcriptome of Rheumatoid Arthritis. Mediators Inflamm. 2022;2022:4766992. doi:10.1155/2022/4766992
65. Qing L, Yujie G, Zhang L, et al. Decreased ALKBH5, FTO, and YTHDF2 in Peripheral Blood Are as Risk Factors for Rheumatoid Arthritis. Biomed Res Int. 2020;2020(1):5735279. doi:10.1155/2020/5735279
66. Jinghua W, Shushan Y, Hongying L, et al. METTL3 Attenuates LPS-Induced Inflammatory Response in Macrophages via NF-κB Signaling Pathway. Mediators Inflamm. 2019;2019:3120391. doi:10.1155/2019/3120391
67. Yazhen S, Zewen W, Yang L, et al. Increased m6A RNA methylation and METTL3 expression may contribute to the synovitis progression of rheumatoid arthritis. Exp Cell Res. 2024;442(2):114237. doi:10.1016/j.yexcr.2024.114237
68. Ziheng Z, Lei W. N6‑methyladenosine methyltransferase METTL14 is associated with macrophage polarization in rheumatoid arthritis. Exp Ther Med. 2024;28(4):375. doi:10.3892/etm.2024.12664
69. Xiaoya C, Fengxia X, Xue P, et al. WTAP-Mediated m6A Modification of TRAIL- DR4 Suppresses MH7A Cell Apoptosis. Int J Rheum Dis. 2025;28(1):e70065. doi:10.1111/1756-185X.70065
70. Lei L, Zhenjuan X, Lei L, et al. WTAP promotes fibroblast-like synoviocyte pyroptosis in Rheumatoid arthritis by upregulating N6-methyladenosine modification of NLRP3. J Bioenerg Biomembr. 2024;56:563–571. doi:10.1007/s10863-024-10035-w
71. Ruiru L, Kuang Y, Yuanyuan N, et al. FTO-mediated RNA mA methylation regulates synovial aggression and inflammation in rheumatoid arthritis. Biochim Biophys Acta Mol Basis Dis. 2024;1870(7):167341. doi:10.1016/j.bbadis.2024.167341
72. Yurong H, Pengfei X, Faxue L, et al. Fat mass and obesity-associated protein inhibit the pathology of rheumatoid arthritis through the NSUN2/SFRP1/Wnt/β-catenin signal axis. J Pharm Pharmacol. 2024;76(3):283–294. doi:10.1093/jpp/rgae003
73. Zhi-Wei F, Chen-Fei Y, He-Fang X, et al. YTHDC1 Regulates the Migration, Invasion, Proliferation, and Apoptosis of Rheumatoid Fibroblast-Like Synoviocytes. Front Immunol. 2024;15:1440398. doi:10.3389/fimmu.2024.1440398
74. Kuang Y, Ruiru L, Jingnan W, et al. ALKBH5 -Mediated RNA m 6 A Methylation Regulates the Migration, Invasion, and Proliferation of Rheumatoid Fibroblast-Like Synoviocytes. Arthritis Rheumatol. 2024;76(2):192–205. doi:10.1002/art.42676
75. Danping F, Qishun G, Bailiang W, et al. Hypoxia-induced ALKBH5 aggravates synovial aggression and inflammation in rheumatoid arthritis by regulating the m6A modification of CH25H. Clin Immunol. 2024;261:109929. doi:10.1016/j.clim.2024.109929
76. Zhaonan B, Zhengjiang L, Shuxing X, et al. IGF2BP3 regulates the expression of RRM2 and promotes the progression of rheumatoid arthritis via RRM2/Akt/MMP-9 pathway. PLoS One. 2024;19(5):e0303593. doi:10.1371/journal.pone.0303593
77. Lei W, Jian L, Chuanbing H, et al. Role of m6A modification and novel circ_0066715/ miR-486-5p/ ETS1 axis in rheumatoid arthritis macrophage polarization progression. Aging (Albany NY). 2022;14(24):10009–10026. doi:10.18632/aging.204439
78. Jianting W, Jian L, Lei W, et al. m6 A-mediated lncRNA MAPKAPK5-AS1 induces apoptosis and suppresses inflammation via regulating miR-146a-3p/SIRT1/NF-κB axis in rheumatoid arthritis. Cell Cycle. 2023;22(23–24):2602–2621. doi:10.1080/15384101.2024.2302281
79. Hongbin L, Jie W, Songye W, et al. Exploring CircRNA N6-methyladenosine in human rheumatoid arthritis: hyper-methylated hsa_circ_0007259 as a potential biomarker and its involvement in the hsa_circ_0007259/hsa_miR-21-5p/STAT3 axis. Int Immunopharmacol. 2023;124:110938. doi:10.1016/j.intimp.2023.110938
80. Yanqiong Z, Xia M, Weijie L, et al. Tripterygium wilfordii: an inspiring resource for rheumatoid arthritis treatment. Med Res Rev. 2021;41(3):1337–1374. doi:10.1002/med.21762
81. Danping F, Bin L, Xiaofeng G, et al. Potential Target Analysis of Triptolide Based on Transcriptome-Wide mA Methylome in Rheumatoid Arthritis. Front Pharmacol. 2022;13:843358. doi:10.3389/fphar.2022.843358
82. Xian L, Cheng T, Ren Z, et al. N6-methyladenosine modification of TGM2 mRNA contributes to the inhibitory activity of sarsasapogenin in rheumatoid arthritis fibroblast-like synoviocytes. Phytomedicine. 2022;95:153871. doi:10.1016/j.phymed.2021.153871
83. Jian C, Xian L, Juan H, et al. Artemisitene suppresses rheumatoid arthritis progression via modulating METTL3-mediated N6-methyladenosine modification of ICAM2 mRNA in fibroblast-like synoviocytes. Clin Transl Med. 2022;12(12):e1148. doi:10.1002/ctm2.1148
84. Arito M, Nagai K, Ooka S, et al. Altered acetylation of proteins in patients with rheumatoid arthritis revealed by acetyl-proteomics. Clin Exp Rheumatol. 2015;33(6):877–886.
85. Xin G, Bingjie G, Xianhui L, et al. 1, 25-dihydroxy-vitamin D3 with tumor necrosis factor-alpha protects against rheumatoid arthritis by promoting p53 acetylation-mediated apoptosis via Sirt1 in synoviocytes. Cell Death Dis. 2016;7(10):e2423. doi:10.1038/cddis.2016.300
86. Youn PS, Won LS, Yeob LS, et al. SIRT1/Adenosine Monophosphate-Activated Protein Kinase α Signaling Enhances Macrophage Polarization to an Anti-inflammatory Phenotype in Rheumatoid Arthritis. Front Immunol. 2017;8:1135. doi:10.3389/fimmu.2017.01135
87. Guoqing L, Zhongbing X, Ying L, et al. SIRT1 inhibits rheumatoid arthritis fibroblast-like synoviocyte aggressiveness and inflammatory response via suppressing NF-κB pathway. Biosci Rep. 2018;38:1.
88. Qing M, Boqi P, Puyi S. Histone deacetylase 1 is increased in rheumatoid arthritis synovium and promotes synovial cell hyperplasia and synovial inflammation in the collagen-induced arthritis mouse model via the microRNA-124-dependent MARCKS-JAK/STAT axis. Clin Exp Rheumatol. 2021;39(5):970–981. doi:10.55563/clinexprheumatol/1xsigp
89. Shao L, Hou C. Hou Chunfeng, miR-138 activates NF-κB signaling and PGRN to promote rheumatoid arthritis via regulating HDAC4. Biochem Biophys Res Commun. 2019;519(1):166–171. doi:10.1016/j.bbrc.2019.08.092
90. Chiara A, Grabiec Aleksander M, Ferguson Bradley S, et al. Inflammatory cytokines epigenetically regulate rheumatoid arthritis fibroblast-like synoviocyte activation by suppressing HDAC5 expression. Ann Rheum Dis. 2016;75(2):430–438. doi:10.1136/annrheumdis-2014-205635
91. Kyun PJ, Sehui S, Jung YH, et al. Inhibition of histone deacetylase 6 suppresses inflammatory responses and invasiveness of fibroblast-like-synoviocytes in inflammatory arthritis. Arthritis Res Ther. 2021;23(1):177. doi:10.1186/s13075-021-02561-4
92. Mozili A, Yongwei Q, Qinge M, et al. The Mechanisms and Quality Markers of Anti-rheumatoid Arthritis of Traditional Chinese Medicine. Comb Chem High Throughput Screen. 2024. doi:10.2174/0113862073321827240918040737
93. Peiyu L, Zhengmin C, Jiayi H, et al. Wutou decoction: a latest review on molecular mechanisms, clinical studies, quality control, pharmacokinetic studies, pharmacological effects, toxicity, and target prediction. J Ethnopharmacol. 2025;341:119307. doi:10.1016/j.jep.2024.119307
94. Pan S, Weiji L, Yao H, et al. Wutou decoction attenuates rheumatoid arthritis in rats through SIRT1-mediated deacetylation of the HMGB1/NF-κB pathway. J Ethnopharmacol. 2025;337:118921. doi:10.1016/j.jep.2024.118921
95. Ying W, Chun Z, Hui G, et al. Therapeutic effect of Cryptotanshinone on experimental rheumatoid arthritis through downregulating p300 mediated-STAT3 acetylation. Biochem Pharmacol. 2017;138:119–129. doi:10.1016/j.bcp.2017.05.006
96. Ah-Reum S, Jung-Yoon Y, KyungChul C, et al. Delphinidin, a specific inhibitor of histone acetyltransferase, suppresses inflammatory signaling via prevention of NF-κB acetylation in fibroblast-like synoviocyte MH7A cells. Biochem Biophys Res Commun. 2011;410(3):581–586. doi:10.1016/j.bbrc.2011.06.029
97. Tapan B, Swati C, Monika S, et al. Ubiquitination in rheumatoid arthritis. Life Sci. 2020;261:118459. doi:10.1016/j.lfs.2020.118459
98. Wen W, Ting X, Yachen Y, et al. E3 ubiquitin ligases STUB1/CHIP contributes to the Th17/Treg imbalance via the ubiquitination of aryl hydrocarbon receptor in rheumatoid arthritis. Clin Exp Immunol. 2022;209(3):280–290. doi:10.1093/cei/uxac072
99. Wen W, Yachen Y, Yujia S, et al. E3 ubiquitin ligase STUB1 affects the mTORC1 pathway through p62 and participates in regulating the differentiation of follicular helper T cells in rheumatoid arthritis. Clin Immunol. 2023;255:109736. doi:10.1016/j.clim.2023.109736
100. Qingliang M, Kai W, Shan Y. BIRC3E3 ubiquitin ligase gene modulates TNF-induced cell death pathways and promotes aberrant proliferation in rheumatoid arthritis fibroblast-like synoviocytes. Front Immunol. 2024;15:1433898. doi:10.3389/fimmu.2024.1433898
101. Lei L, Geng Z, Lei L, et al. USP2 Promotes the Proliferation and Inflammation of Fibroblast-Like Synovial Cells in Rheumatoid Arthritis Through Deubiquitination of TRAF2. Biochem Genet. 2025;63:592–605. doi:10.1007/s10528-024-10737-1
102. Liman L, Zhiwen H, Wenjuan L, et al. Mid1 promotes synovitis in rheumatoid arthritis via ubiquitin-dependent post-translational modification. Pharmacol Res. 2024;205:107224. doi:10.1016/j.phrs.2024.107224
103. Man-Yu Z, Meng-Qing W, Yan H, et al. Silencing aquaporin 1 inhibits autophagy to exert anti-rheumatoid arthritis effects in TNF-α-induced fibroblast-like synoviocytes and adjuvant-induced arthritis rats. Inflamm Res. 2025;74(1):12. doi:10.1007/s00011-024-01966-6
104. Yujun R, Yuxuan F, Wei T, et al. Delivery of Long Non-coding RNA NEAT1 by Peripheral Blood Monouclear Cells-Derived Exosomes Promotes the Occurrence of Rheumatoid Arthritis via the MicroRNA-23a/MDM2/SIRT6 Axis. Front Cell Dev Biol. 2020;8:551681. doi:10.3389/fcell.2020.551681
105. Xiaolong S, Shaomin C, Jinti L, et al. Knockdown of lncRNA NEAT1 inhibits Th17/CD4 +T cell differentiation through reducing the STAT3 protein level. J Cell Physiol. 2019;234(12):22477–22484. doi:10.1002/jcp.28811
106. Xiaoran S, Xinliu W, Xin Z, et al. β-Indole-3-acetic acid attenuated collagen-induced arthritis through reducing the ubiquitination of Foxp3 via the AhR-TAZ-Tip60 pathway. Immunol Res. 2024;72(4):741–753. doi:10.1007/s12026-024-09480-x
107. Wenjing Y, Yongwang Z, Xin H, et al. Auranofin targets UBA1 and enhances UBA1 activity by facilitating ubiquitin trans-thioesterification to E2 ubiquitin-conjugating enzymes. Nat Commun. 2023;14(1):4798. doi:10.1038/s41467-023-40537-x
108. Xiangjie C, Qiuyu W, Xinhua C, et al. Menthone inhibits type-I interferon signaling by promoting Tyk2 ubiquitination to relieve local inflammation of rheumatoid arthritis. Int Immunopharmacol. 2022;112:109228. doi:10.1016/j.intimp.2022.109228
109. Pei-Rong G, Hong W, Yu-Long Z, et al. Glycolysis, a driving force of rheumatoid arthritis. Int Immunopharmacol. 2024;132:111913. doi:10.1016/j.intimp.2024.111913
110. Qianwei L, Yuehong C, Huan L, et al. Targeting glycolytic pathway in fibroblast-like synoviocytes for rheumatoid arthritis therapy: challenges and opportunities. Inflamm Res. 2023;72(12):2155–2167. doi:10.1007/s00011-023-01807-y
111. Maryam M, Hamidreza B, Hossein K, et al. Destructive Roles of Fibroblast-like Synoviocytes in Chronic Inflammation and Joint Damage in Rheumatoid Arthritis. Inflammation. 2021;44(2):466–479. doi:10.1007/s10753-020-01371-1
112. Jianlin Z, Jinshuo T, Meng L, et al. Glycolysis Rate-Limiting Enzymes: novel Potential Regulators of Rheumatoid Arthritis Pathogenesis. Front Immunol. 2021;12:779787. doi:10.3389/fimmu.2021.779787
113. Chi S, Xinhui Z, Tao T, et al. The β4GalT1 affects the fibroblast-like synoviocytes invasion in rheumatoid arthritis by modifying N-linked glycosylation of CXCR3. Eur J Cell Biol. 2017;96(2):172–181. doi:10.1016/j.ejcb.2017.02.001
114. Dongying C, Xiaoyan C, Hui O, et al. Increased eEF2K Promotes Glycolysis and Aggressive Behaviors of Fibroblast-Like Synoviocytes in Rheumatoid Arthritis. J Inflamm Res. 2022;15:1729–1744. doi:10.2147/JIR.S337620
115. Mei Z, Ning L, Xiao-Yun G, et al. Influences of the lncRNA TUG1-miRNA-34a-5p network on fibroblast-like synoviocytes (FLSs) dysfunction in rheumatoid arthritis through targeting the lactate dehydrogenase A (LDHA). J Clin Lab Anal. 2021;35:e23969. doi:10.1002/jcla.23969
116. Bonah K, Young KH, Ruem YB, et al. Cytoplasmic zinc promotes IL-1β production by monocytes and macrophages through mTORC1-induced glycolysis in rheumatoid arthritis. Sci Signal. 2022;15(716):eabi7400. doi:10.1126/scisignal.abi7400
117. Katrien VR, Sadiq U, Karol P, et al. Interleukin-34 Reprograms Glycolytic and Osteoclastic Rheumatoid Arthritis Macrophages via Syndecan 1 and Macrophage Colony-Stimulating Factor Receptor. Arthritis Rheumatol. 2021;73(11):2003–2014. doi:10.1002/art.41792
118. Weiwei C, Jingwen C, Shiye Z, et al. The glycolysis inhibitor 2-deoxyglucose ameliorates adjuvant-induced arthritis by regulating macrophage polarization in an AMPK-dependent manner. Mol Immunol. 2021;140:186–195. doi:10.1016/j.molimm.2021.10.007
119. Dan-Dan W, Chi-Yi H, Yi-Jin W, et al. AMPK/SIRT1 Deficiency Drives Adjuvant-Induced Arthritis in Rats by Promoting Glycolysis-Mediated Monocytes Inflammatory Polarization. J Inflamm Res. 2022;15:4663–4675. doi:10.2147/JIR.S378090
120. Jing X, Congshan J, Xipeng W, et al. Upregulated PKM2 in Macrophages Exacerbates Experimental Arthritis via STAT1 Signaling. J Immunol. 2020;205(1):181–192. doi:10.4049/jimmunol.1901021
121. Sadiq U, Karol P, Volin Michael V, et al. IRAK4 inhibitor mitigates joint inflammation by rebalancing metabolism malfunction in RA macrophages and fibroblasts. Life Sci. 2021;287:120114. doi:10.1016/j.lfs.2021.120114
122. Jingjing Q, Jiaqing L, Xiangge Z, et al. IL-27 enhances peripheral B cell glycolysis of rheumatoid arthritis patients via activating mTOR signaling. Int Immunopharmacol. 2023;121:110532. doi:10.1016/j.intimp.2023.110532
123. Shilpa H, Poulami D, Srivatsan R. Altered Transcriptional Regulation of Glycolysis in Circulating CD8 T Cells of Rheumatoid Arthritis Patients. Genes. 2022;13:1.
124. Yuan D, Panwang L, Wen W, et al. Sarsasapogenin, a principal active component absorbed into blood of total saponins of Anemarrhena, attenuates proliferation and invasion in rheumatoid arthritis fibroblast-like synoviocytes through downregulating PKM2 inhibited pathological glycolysis. Phytother Res. 2023;37(5):1951–1967. doi:10.1002/ptr.7712
125. Mei-Yu S, Xiang W, Yu-Xi D, et al. Triptolide inhibits Th17 differentiation via controlling PKM2-mediated glycolysis in rheumatoid arthritis. Immunopharmacol Immunotoxicol. 2022;44(6):838–849. doi:10.1080/08923973.2022.2086139
126. Qiqi Y, Haixia L, Shiyue S, et al. Adipose-derived stem cell exosomes loaded with icariin alleviates rheumatoid arthritis by modulating macrophage polarization in rats. J Nanobiotechnology. 2024;22(1):423. doi:10.1186/s12951-024-02711-1
127. Chenyang L, Rui-Juan C, Qiuping Z, et al. Herbal compound cepharanthine attenuates inflammatory arthritis by blocking macrophage M1 polarization. Int Immunopharmacol. 2023;125:111175. doi:10.1016/j.intimp.2023.111175
128. Jing-Wen C, Yun Y, Shi-Ye Z, et al. Berberine ameliorates collagen-induced arthritis in mice by restoring macrophage polarization via AMPK/mTORC1 pathway switching glycolytic reprogramming. Int Immunopharmacol. 2023;124:111024. doi:10.1016/j.intimp.2023.111024
129. Tian-Tian J, Chao-Fan J, Xiu-Ping C, et al. α-Mangostin Alleviated HIF-1α-Mediated Angiogenesis in Rats With Adjuvant-Induced Arthritis by Suppressing Aerobic Glycolysis. Front Pharmacol. 2021;12:785586. doi:10.3389/fphar.2021.785586
130. Yue Y, Jinjie S, Jiming Y, et al. New Posttranslational Modification Lactylation Brings New Inspiration for the Treatment of Rheumatoid Arthritis. J Inflamm Res. 2024;17:11845–11860. doi:10.2147/JIR.S497240
131. Weicong F, Tianbao W, Yehong L, et al. The role of lactylation in plasma cells and its impact on rheumatoid arthritis pathogenesis: insights from single-cell RNA sequencing and machine learning. Front Immunol. 2024;15:1453587. doi:10.3389/fimmu.2024.1453587
132. Yan-Hang W, Peng G, Yu-Qi W, et al. Small-molecule targeting PKM2 provides a molecular basis of lactylation-dependent fibroblast-like synoviocytes proliferation inhibition against rheumatoid arthritis. Eur J Pharmacol. 2024;972:176551. doi:10.1016/j.ejphar.2024.176551
133. Yunkai Z, Ying G, Yujia W, et al. RBM25 is required to restrain inflammation via ACLY RNA splicing-dependent metabolism rewiring. Cell Mol Immunol. 2024;21(11):1231–1250. doi:10.1038/s41423-024-01212-3
134. Arévalo-Caro C, Romero-Sánchez C, Garavito-Rodríguez E. Relation between anti-Porphyromonas gingivalis antibody titers and HLA-DRB1 neutral alleles in individuals with rheumatoid arthritis. Acta Odontologica Scandinavica. 2022;80(2):131–139. doi:10.1080/00016357.2021.1959053
135. He Y, Ge C, Moreno-Giró À, et al. A subset of antibodies targeting citrullinated proteins confers protection from rheumatoid arthritis. Nature Communications. 2023;14(1):691. doi:10.1038/s41467-023-36257-x
136. Alghamdi MA, Redwan EM. Interplay of microbiota and citrullination in the immunopathogenesis of rheumatoid arthritis. Probiotics and Antimicrobial Proteins. 2022;14(1):99–113. doi:10.1007/s12602-021-09802-7
137. Zhao Q, Yu J, Zhou H, et al. Intestinal dysbiosis exacerbates the pathogenesis of psoriasis-like phenotype through changes in fatty acid metabolism. Signal Transduction and Targeted Therapy. 2023;8(1):40. doi:10.1038/s41392-022-01219-0
138. Shi J, van Steenbergen HW, van Nies JAB, et al. The specificity of anti-carbamylated protein antibodies for rheumatoid arthritis in a setting of early arthritis. Arthritis Research & Therapy. 2015;17(1):1–6. doi:10.1186/s13075-015-0860-6
139. Reed E, Jiang X, Kharlamova N, et al. Antibodies to carbamylated α-enolase epitopes in rheumatoid arthritis also bind citrullinated epitopes and are largely indistinct from anti-citrullinated protein antibodies. Arthritis Research & Therapy. 2016;18(1):1–9. doi:10.1186/s13075-016-1001-6
140. Chen X, Du R, Wang P, et al. Proteomic analysis of infiltrating neutrophils from rheumatoid arthritis synovial fluid and their contribution to protein carbamylation. Frontiers in Immunology. 2025;16:1563426. doi:10.3389/fimmu.2025.1563426
141. O’Neil LJ, Oliveira CB, Wang X, et al. Neutrophil extracellular trap-associated carbamylation and histones trigger osteoclast formation in rheumatoid arthritis. Annals of the Rheumatic Diseases. 2023;82(5):630–638. doi:10.1136/ard-2022-223568
142. Shu F, Xiao H, Li QN, et al. Epigenetic and post-translational modifications in autophagy: biological functions and therapeutic targets. Signal Transduction and Targeted Therapy. 2023;8(1):32. doi:10.1038/s41392-022-01300-8
143. Riitano G, Recalchi S, Capozzi A, et al. The role of autophagy as a trigger of post-translational modifications of proteins and extracellular vesicles in the pathogenesis of rheumatoid arthritis. International Journal of Molecular Sciences. 2023;24(16):12764. doi:10.3390/ijms241612764
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
New Posttranslational Modification Lactylation Brings New Inspiration for the Treatment of Rheumatoid Arthritis
Yang Y, Shi J, Yu J, Zhao X, Zhu K, Wang S, Zhang X, Zhang X, Wei G, Cao W
Journal of Inflammation Research 2024, 17:11845-11860
Published Date: 30 December 2024

