Back to Journals » Journal of Inflammation Research » Volume 17
The Role of Chitinase 3-Like-1 (YKL-40) and Proinflammatory Biomarkers in the Pathogenesis of Pediatric Tick-Borne Encephalitis in a Polish Cohort
Authors Bojkiewicz E , Toczylowski K , Lewandowski D, Martonik D, Flisiak R, Sulik A
Received 30 May 2024
Accepted for publication 17 November 2024
Published 5 December 2024 Volume 2024:17 Pages 10239—10254
DOI https://doi.org/10.2147/JIR.S480556
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam Bachstetter
Ewa Bojkiewicz,1 Kacper Toczylowski,1 Dawid Lewandowski,1 Diana Martonik,2 Robert Flisiak,2 Artur Sulik1
1Department of Pediatric Infectious Diseases, Medical University of Bialystok, Bialystok, Poland; 2Department of Infectious Diseases and Hepatology, Medical University of Bialystok, Bialystok, Poland
Correspondence: Kacper Toczylowski, Email [email protected]
Background: Chitinase 3-like-1 (CHI3L1), also known as YKL-40, is a potential biomarker for neuroinflammatory conditions. It is upregulated in Alzheimer’s disease, multiple sclerosis, and traumatic brain injury. However, its involvement in pediatric tick-borne encephalitis (TBE) has not been addressed yet. This study aimed to evaluate CHI3L1 and its relationship with other inflammatory cytokines, blood-brain barrier (BBB) integrity, immune response, and disease severity in pediatric patients with TBE.
Patients and Methods: A total of 22 pediatric TBE patients hospitalized in Bialystok, Poland were included in this study. Participants were categorized as having meningoencephalitis (n=6) or meningitis (n=16). The integrity of the brain-blood barrier (BBB) was assessed using the albumin quotient (albQ). Biomarker indices were calculated to account for variations in BBB permeability. The concentrations of CHI3L1, CCL2, chemerin, CXCL2, IFN-γ, IL-1-β, IL-4, IL-6, IL-13, and TNF-α in both serum and CSF, were measured using the Luminex Multiplex Assay od admission and two weeks later when symptoms resolved.
Results: CSF and serum concentrations of CHI3L1 did not differ between the encephalitis and meningitis cases. After adjusting for BBB permeability, the CHI3L1 index was 2.4-fold lower in patients with encephalitis than in those with meningitis (P=0.008). There was a post-treatment reduction of CHI3L1, IL-6, and TNF-α CSF concentrations. We also found and improvement in BBB permeability in younger children but in older albQ remained abnormal. Correlation analysis revealed associations between CHI3L1 levels and pro-inflammatory markers, notably chemerin, IL-6, and TNF-α, across both clinical groups.
Conclusion: Our findings suggest that CHI3L1 CSF levels reflect the inflammatory activity in pediatric TBE and may help to differentiate between meningoencephalitis and meningitis. The observed interactions between CHI3L1 and other cytokines underscore its potential involvement in inflammatory response to the virus. The prolonged disruption in BBB integrity in older children might reflect age-dependent differences in the severity of TBE. These insights advance our understanding of TBE pathogenesis in children and support further investigation of CHI3L1 as a biomarker for TBE diagnosis and management.
Keywords: chitinase-3-like-1, tick-borne encephalitis, blood-brain barrier, ticks, chemokines
Introduction
Tick-borne encephalitis (TBE) is one of the most prevalent viral infections of the central nervous system (CNS) in Poland and is caused by the tick-borne encephalitis virus (TBEV).1 This virus predominantly spreads through infected ticks of the genus Ixodes and can culminate in severe sequelae. The mortality rate for TBE vary depending on the virus subtype. Case-fatality rate is estimated to be less than 2% for European and Siberian subtype, while infections with Far Eastern TBEV subtype have a mortality rate of 20%-40%.2 Reports indicate that 20–58% of patients suffer from complications such as spinal nerve paresis, hearing impairment, dysarthria, or mental disorders.2,3 The recovery time for TBE can varies depending on the severity of the infection. In general, it may take several weeks to 12 months for a complete recovery, especially in cases with neurological involvement. While it has been proven that the severity of complications continue to diminish beyond a year after the acute illness, some individuals experience post - encephalitic syndrome (PES) that can become permanent, with some individuals not achieving full recovery even 15 years after infection.4 TBEV infections have been on the rise across Europe, with Poland experiencing an annual caseload ranging from 150 to 283, predominantly concentrated in the northeastern region.5
Diagnosing TBE relies on clinical symptoms and the detection of specific antibodies in both serum and cerebrospinal fluid (CSF), which are present in general in 93% of patients.6 While CSF characteristics can vary depending on the disease stage and the patient there are some characteristic findings. Reports show that approximately 70–90% of patients may have elevated albumin concentrations. Higher albumin CSF/serum quotient, along with elevated IgG index, are observed in a similar proportion of cases, roughly in the range of 60–80% and 80–90% of cases respectively.7 Additionally, as with other neurotropic Flaviviruses such as West Nile virus (WNV) and Japanese encephalitis virus (JEV), initial diagnostic examinations may reveal a predominance of neutrophils in the CSF leukocyte population.8–10
Disruption of the blood-brain barrier (BBB) and the ensuing intrathecal humoral response are pivotal facets of TBE pathogenesis. Although the precise mechanisms by which the TBE virus compromises the BBB remain elusive, it is postulated that the virus may directly infect and impair endothelial cells, thereby increasing BBB permeability and facilitating the ingress of viruses and immune cells into the brain.9,10 Additionally, inflammatory molecules, including cytokines and chemokines such as IFNγ, TNFα, IL-6, IL-12, IL-1β, CCL-2, CCL-3, CXCL-10, may fuel immune responses in the brain, exacerbating tissue damage and the manifestation of neurological symptoms.11,12
Chitinase 3-like-1 (CHI3L1), also known as YKL-40, has emerged as a potential marker of various neuroinflammatory processes and plays a pivotal role in mediating inflammation and immune responses. Elevated CHI3L1 expression has been noted in conditions such as Alzheimer’s disease, multiple sclerosis, and traumatic brain injury, suggesting its involvement in the pathogenesis and progression of these disorders.13,14 Dorcet et al have described how CHI3L1 could be a valuable biomarker to follow autoimmune encephalitis activity and in consequence supports the clinician’s therapeutic decision in questionable cases.15 However, the exact mechanisms and therapeutic implications of CHI3L1 in other brain pathologies remain unclear.
CHI3L1, a glycoprotein belonging to the family of 18 glycosyl hydrolases, is primarily secreted by activated neutrophils and macrophages. Within the central nervous system, microglia, especially in response to acute and chronic inflammation, serve as the primary source of CHI3L1 secretion.16 It has been proposed that other intrathecal chemokines mediate and balance immune responses in brain cells. Previous research suggests that CHI3L1 production is influenced by various cytokines and chemokines, including IFN-γ, TNF-α, IL-4, IL-6, IL-13, CCL2, CXCL2, and chemerin.17–20 However, unresolved questions remain regarding the dynamics of protein production during the course of TBE and their potential beneficial or deleterious effects. Hence, this study aimed to analyze the role of CHI3L1 in the pathogenesis of TBE. We also sought to investigate if CHI3L1 was connected to the severity of TBE, the disruption of the BBB and to investigate relationships between CHI3L1 and selected pro- and anti-inflammatory factors.
Material and Methods
Twenty-two children diagnosed with tick-borne encephalitis (TBE) and hospitalized between June 2020 and August 2022 in the Department of Pediatric Infectious Diseases in Bialystok, Poland were included in this study. None of the patients had a history of TBE vaccination and all resided in Podlaskie Voivodeship, where the European subtype of TBEV is endemic. The biphasic course of the infection was recorded in 19 children, the remaining three reported monophasic infection. The median duration of neurological symptoms before lumbar puncture was three days (IQR, 3–4 days).
The patients were divided into two groups: meningoencephalitis (n=6) and meningitis (n=16). Meningoencephalitis was diagnosed when inflammatory parameters in the CSF, altered consciousness, and focal neurological symptoms were present, whereas meningitis was diagnosed on the basis of inflammatory parameters in the CSF without focal neurological symptoms. TBE was diagnosed based on the presence of specific anti-TBEV IgG and IgM antibodies in serum and/or CSF detected with commercially available enzyme-linked immunosorbent assays, meeting the diagnostic criteria of the European Academy of Neurology.7
TBE severity was assessed on admission to the hospital using a numeric scale and standardized questionnaire according to a paper published in 2014 by Petra Bogovic.21 This scale was originally used for adult populations. However, due to the lack of studies on pediatric populations, we decided to implement this scale in our analysis. A certain number of points (from 1 to 9) were assigned for the presence, intensity, and duration of each individual symptom, such as the presence and duration of headache, fever, vomiting, and meningeal signs; presence of tremor, pareses, urine retention, and cognitive function disturbances; presence and intensity of conscious disturbances; and need for and duration of treatment of elevated intracranial pressure. Clinically, mild, moderate, and severe disease courses correspond to scores of 0–8, 9–22, and >22, respectively.
Luminex assays (R&D Systems, Minneapolis, MN, USA) were used for the quantitative assessment of CHI3L1, Chemokine (C-C motif) ligand 2 (CCL-2), chemokine (C-X-C motif) ligand 2 (CXCL2), chemerin, interferon gamma (IFN-γ), interleukin 1 beta (IL-1β), IL-4, IL-6, IL-13, and tumor necrosis factor alpha (TNF-α) levels in both serum and CSF samples. Serum and CSF samples were taken on the same day from each included patient twice: on admission (T1) and 14 days after symptom resolution (T2). All collected samples were immediately stored at −80°C before analysis.
Assays were conducted according to the manufacturer’s instructions using the Bio-Plex 200 System (Bio-Rad Laboratories, Hercules, CA, USA). This multiplex immunoassay system facilitates simultaneous detection of multiple analytes within a single serum sample. Prior to analysis, serum samples were subjected to processing and storage following standardized protocols to maintain sample integrity. Quality control measures were implemented to ensure precision and reproducibility of the assay outcomes. These measures included the utilization of standard reference materials and the generation of calibration curves. All assay methodologies and procedures strictly adhered to both the manufacturer’s guidelines and established laboratory protocols.
Concentrations of measured cytokines and chemokines was further corrected to the permeability of the BBB. BBB permeability was assessed using the albumin quotient (Q) and the concentrations of the analyzed proteins in the CSF were adjusted accordingly. Calculated albumin Q levels where compared to the normal values.22 The CSF/serum quotient was calculated by dividing the concentration of each cytokine or chemokine in the CSF by that in the serum (biomarker in CSF/biomarker in serum). The CSF/serum index was defined as the ratio of the quotients of each studied chemokine or cytokine to that of albumin (Q-biomarker/Q-albumin).
Statistical Analysis
All analyses were conducted using the R Statistical language (version 4.1.1; R Core Team, 2021) on Windows 10 pro 64 bit (build 19045), using the package effectsize (version 0.8.323), rio (version 0.5.2924), report (version 0.5.725), ggstatsplot (version 0.9.326), gtsummary (version 1.6.227), cutpointr (version 1.1.228), readxl (version 1.3.129), and dplyr (version 1.1.230). The significance level of the statistical tests in this analysis was set at α=0.05. The normality of the distributions of the variables was analyzed using the Shapiro–Wilk test. Numerical variables with distributions deviating from the normal distribution were reported as medians (Mdn) with interquartile range (Q1, Q3). Categorical variables were reported as counts (n) and percentages (%).The differences between two groups were assessed using the Wilcoxon rank-sum test, with effect size estimated using the rank-biserial correlation ( ). Effect sizes were interpreted based on Funder’s Convention. The significance of the association between the two numerical variables was determined using Spearman correlation coefficient. The p-values were computed using the AS 89. Determination and evaluation of optimal cutpoints for CHI3L1 between disease group severity levels were performed in a robust manner using the maximization metric method (sum of specificity and sensitivity) with more or equal (“≥”) direction with bootstrapping procedures. Out-of-sample variability and power were assessed using 1000 bootstrap samples. A sample of the same size as the original data was drawn from the original data and replaced. Cut-point estimation was performed for the in-bag sample, and the determined cut-off point was applied to the in-bag and out-of-bag observations.
). Effect sizes were interpreted based on Funder’s Convention. The significance of the association between the two numerical variables was determined using Spearman correlation coefficient. The p-values were computed using the AS 89. Determination and evaluation of optimal cutpoints for CHI3L1 between disease group severity levels were performed in a robust manner using the maximization metric method (sum of specificity and sensitivity) with more or equal (“≥”) direction with bootstrapping procedures. Out-of-sample variability and power were assessed using 1000 bootstrap samples. A sample of the same size as the original data was drawn from the original data and replaced. Cut-point estimation was performed for the in-bag sample, and the determined cut-off point was applied to the in-bag and out-of-bag observations.
Results
Characteristics of the Studied Population
Our study included 22 pediatric patients (seven girls and 15 boys) hospitalized for TBE between June 2020 and August 2022. The detailed demographic and clinical characteristics are summarized in Table 1. The cohort was divided based on clinical diagnosis: 6 with meningoencephalitis (1 female, 5 males) and 16 with meningitis (6 females, 10 males). Disturbances in consciousness were exclusive to the encephalitis subset. Neurological manifestations included tremor (12/22), unsteady gait (1/22), photophobia (5/22), emotional lability (3/22), and generalized seizures (2/22). On average, all participants experienced headaches lasting 6 days. Fever was noted in of the 21/22 subjects with an average duration of 6 days (range 10–23 days), and vomiting was reported in 17 patients. Treatment protocols included mannitol (15%) administered 2–4 times daily for 3–5 days, complemented by NSAIDs, as required by European Academy of Neurology.7 The median length of the hospital stay was 17.5 days. Laboratory assessments revealed normal or slightly elevated levels of CRP and white blood cells, with neutrophils predominating in most cases. On the basis of the numerical disease severity scale, most cases were moderate (15/22), with the remainder classified as mild (4/22) or severe (3/22). None of the patients experienced long-term complications.
 |
Table 1 Sociodemographic and Clinical Characteristics of Pediatric Patients Hospitalized with Tick-Borne Encephalitis (TBE) |
Pre-post treatment differences in concentrations of chitinase-3-like 1 and other cytokines in the CSF and serum
Protein detectability varied, with some cytokines, such as IFN-γ, IL-4, IL-13, and IL-1 beta only occasionally detectable in the serum and more frequently in the CSF. Regarding the serum samples, IFN-γ was detected in one T1 sample and two T2 samples only, IL-4 in three T1 samples and one T2 sample, and IL-13 and IL-1 beta were undetectable in both T1 and T2 serum samples. The detectability of proteins in CSF samples was higher: IL-13 was detected in one patient on T1 and two on T2, IL-1 beta was detected in three patients on T1, and none on T2. While CXCL-2 was detectable in all serum samples and 12 T1 CSF samples, it was detected in three CSF samples collected on T2 only. This limits the calculation of CSF/serum quotients and indices. We could not calculate CSF/serum quotients and indices for CXCL2 and IL-4 on T2, IL-1 beta, IL-13 and INF-γ on both T1 and T2. The baseline and posttreatment protein concentrations are shown in Table 2.
 |
Table 2 Serum and Cerebrospinal Fluid Biomarker Levels Upon Admission and After Two Weeks of Treatment in Pediatric TBE Patients |
Significant reductions in CSF CHI3L1 levels were observed post-treatment (p=0.009), especially in meningitis but not in encephalitis (Figures 1 and 2). However, serum CHI3L1 levels did not exhibit statistically significant changes. Similarly, no significant differences were observed in the CHI3L1 CSF/serum quotient and index values between the two time points.
Additionally, a greater decrease in CHI3L1 serum level at the time point after 2 weeks was significantly correlated with more severe headache (rho = −0.46, p =0.031, npairs = 22), while a significantly greater decrease in CSF was observed among girls (median = −1.84×103 (Q1 = −3.68×103, Q3 = 0.67×103) compared to boys (median = −1.56×103 (Q1 = −2.39×103, Q3 = 1.49×103), p = 0.047, n = 22). Second, we observed a significant correlation between a greater decrease in the CHI3L1 quotient at the time point after two weeks with a longer intake of mannitol (rho = −0.45, p =0.034, npairs = 22). Finally, a greater decrease in the CHI3L1 index value after treatment was significantly correlated with shorter hospitalizations (rho = 0.47, p =0.026, npairs = 22).
The levels of other cytokines in the CSF, including IL-6 and TNF-α, decreased post-treatment. There was also a decreasing trend in the CCL-2 and chemerin concentrations in the CSF. Serum concentrations of these proteins were inconsistent: CCL-2 increased (p=0.017) and IL-6 decreased (p=0.004); serum concentrations of chemerin and CXCL2 did not change over the course of two weeks. There was however a significant reduction of CCL-2 Q and index values, as well as IL-6 Q and index, and TNF-α Q. We found a correlation between a greater decrease in the IL-6 quotient and index after two weeks with a longer intake of mannitol (respectively: rho = −0.57, p =0.006, npairs = 21; and rho = −0.52, p =0.016, npairs = 21). The decrease of CCL2 and TNF-α markers were not correlated with clinical presentation.
Severity-Dependent Variations in CHI3L1 Levels
The CHI3L1 index values differed significantly between encephalitis and meningitis, with lower levels in encephalitis (Table 3). Lower values were also found for the CHI3L1 quotient in encephalitis, but only at the trend level (p<0.10). It can be assumed that this result was probably due to the increased albumin quotient in encephalitis cases. Nevertheless, a large effect size was obtained in both cases (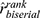 = 0.73 and 0.54 respectively). There were no significant differences in the levels of the CHI3L1 in CSF and serum, quotient, or index values according to the severity scale.
= 0.73 and 0.54 respectively). There were no significant differences in the levels of the CHI3L1 in CSF and serum, quotient, or index values according to the severity scale.
Regarding other analyzed cytokines, there was a strong negative correlation between IL-4 in CSF and the numerical severity score (rho= –0.63; p=0.014). Concentrations of other cytokines in the CSF and serum, as well as their quotient and index values, did not differ between the severity groups and did not correlate with the disease severity.
CHI3L1 index discriminated between encephalitis and meningitis. The calculated cutoff value of 0.95 for the chitinase 3-like-1 index suggests that individuals with a value of 0.95 or less are at high risk for tick-borne encephalitis, with strong sensitivity and specificity metrics (Table 4). Conversely, individuals with values greater than 0.95 were classified as being at low risk for encephalitis. In contrast, the estimated cutoff value for CHI3L1 Q did not allow for a good differentiation of patients with encephalitis.
Interconnections Between CHI3L1 and Other Variables
The correlation analysis highlighted in Table 5 provides key insights into the interplay between CHI3L1 and other biomarkers, underscoring its significant role in the neuroinflammatory processes characteristic of TBE. Notably, CHI3L1 levels in the serum and CSF exhibited diverse correlation patterns with biomarkers, such as albumin, chemerin, IL-6, and TNF-α. For instance, a strong negative correlation between CHI3L1 serum levels and serum albumin (rho = −0.39, p = 0.008) contrasts with the positive correlations observed with the same biomarkers in the CSF. Additionally, positive correlations of CHI3L1 in the CSF with pro-inflammatory cytokines such as IL-6 (rho = 0.43, p = 0.004) and TNF-α (rho = 0.31, p = 0.039), and with the chemokine chemerin (rho = 0.42, p = 0.005), highlighted its involvement in mediating inflammatory responses within the central nervous system. A detailed correlation analysis is provided in the supplementary files.
 |
Table 5 Spearman Correlation Coefficients Between Chitinase-3-Like 1 Protein and Other Measured Parameters in Serum and CSF in Pediatric TBE Patients |
Brain-Blood Barrier Permeability in TBE
Elevated albumin Q levels, indicating a disrupted BBB permeability, were observed in 16 children (10/16 children with meningitis and 6/6 children presenting as encephalitis). Median albumin Q on admission was 6.40 (IQR; 4.9, 8.3). When the symptoms resolved this marker dropped to median 4.6 (IQR; 3.3, 7.5), but the difference did not reach statistical significance (p=0.067). In children diagnosed with encephalitis, the median albumin quotient decreased from 8.8 (interquartile range [IQR] 6.8–11.7) at T1 to 4.9 (IQR 3.0–8.1) at T2 (p=0.046). This decrease indicates an improvement in BBB integrity over the course of treatment. In contrast, in meningitis cases, the albumin Q values remained constant between the two time points. This is because albumin Q normalized at T2 in 10 of 16 children with elevated albumin Q on T1 (that includes 7 meningitis and 3 encephalitis cases), Albumin Q remained elevated in the remaining 6 children (3 meningitis and 3 encephalitis cases). In 3 children with normal albumin Q on T1, it remained normal on T2. In the other 3 children albumin Q increased on T2, despite being normal on T1.
Normalization of albumin Q was associated with younger age (median 6.3; IQR, 4.4, 12.8 vs median 16.0 years; IQR, 14.3, 16.9; p=0.034), lower IL-6 Q on T1 (median 39.9; IQR, 20.5, 112.6; vs median, 401.3; IQR, 110.6, 609.7; p=0.01), and lower IL-6 index (median 6.2; IQR, 2.2, 12.7 vs median, 42.7; IQR, 16.0, 59.9; p=0.007), There were no differences in clinical presentation and concentrations of other chemokines and cytokines between children who have and have not normalized albumin Q after treatment.
Children diagnosed with encephalitis had a higher albumin Q than those diagnosed with meningitis (p=0.032), indicating increased BBB permeability. In order to further investigate BBB permeability in TBE we created the correlation matrix. The analysis revealed that CHI3L1 serum levels and CHI3L1 index values (but not CSF CHI3L1 concentrations) were positively correlated with albumin Q (p=0.007 and p<0.001, respectively). Among the CSF cytokines, there were strong positive correlations between the albumin Q and IL-4, IL-6, and TNF-α (see Supplementary Table 1).
Discussion
TBE is a significant public health concern, particularly in regions where the causative virus is endemic. Pediatric cases of TBE present unique challenges owing to the developing nature of the central nervous system and potential for long-term neurological sequelae. In recent years, researchers have explored the utility of biomarkers for enhancing the diagnosis, prognosis, and treatment monitoring of TBE. Cytokines and chemokines are small proteins that play crucial roles in cell signaling and communication within the immune system. They are involved in various physiological processes including inflammation and immune responses. One of the biomarkers for TBE is chitinase 3-like-1.
CHI3L1, also known as human cartilage glycoprotein-39 (HC-gp39) or YKL-40, is a glycoprotein secreted by various cell types including macrophages, neutrophils, and chondrocytes in response to inflammation and tissue remodeling.31 Its involvement in neuroinflammatory processes has sparked interest in its potential as a biomarker for neurological disorders including TBE.
In viral infections affecting the central nervous system, various cytokines and chemokines limit viral replication and ultimately control the spread of the virus. However, the accumulation of proinflammatory mediators in the CNS may also result in progressing the severity of encephalitis and consequently cause permanent neuronal damage.17,32,33 In the context of TBE, elevated CHI3L1 levels may reflect the extent of neuroinflammation and neuronal damage, thereby providing valuable insight into disease pathogenesis and progression. Recent studies have indicated that CHI3L1 plays a major role in tissue injury, inflammation, repair, and remodeling responses. It has been strongly associated with diseases including asthma, arthritis, sepsis, diabetes, liver fibrosis, coronary artery disease, ischemic stroke and Alzheimer’s disease, and could even provide a useful inflammatory marker for patients with toxoplasmosis or Kawasaki disease. More importantly, CHI3L1 has been proven to be independently associated with poor outcomes, disease severity, progression, and an unfavorable course of these diseases.34–38
In the current study, our main goal was to examine the role of CHI3L1 in TBE in children and to assess the influence of various pro- and anti-inflammatory cytokines that could affect the production of CHI3L1. While some children experience mild symptoms and recover fully, others develop severe neurological complications including meningitis, encephalitis, and long-term cognitive impairment. However, the underlying factors that contribute to this variability remain poorly understood. Biomarkers such as CHI3L1 offer potential means for elucidating these mechanisms. Czupryna et al discovered that CHI3L1 concentration in the CSF of adult patients with TBE was significantly higher in patients with meningitis and was associated with a milder course of the disease. They concluded that this biomarker was not useful for the prognosis of sequelae development, as both serum and CSF CHI3L1 concentrations were comparable in patients who recovered completely and in those with an unfavorable course.39 In our study, significant reductions in CSF CHI3L1 levels were observed post-treatment, which were especially pronounced in meningitis cases. We showed significantly lower CHI3L1 index values in encephalitis cases than in meningitis cases, which suggests that CHI3L1 is associated with a milder course of TBE in children. Additionally, a greater decrease in CHI3L1 serum levels post-treatment was significantly correlated with more severe headache and, consequently, a longer intake of mannitol. A greater decrease in CHI3L1 index after treatment was significantly correlated with shorter hospitalization, which could indicate that CHI3L1 play a major role in the recovery process.
In our study, we also showed that a wide range of cytokines and chemokines were produced in response to TBEV infection. It has been shown that in the pediatric population, concentrations of particularly IL-6, TNF-α, and IFN-γ increase in CSF or serum in CNS infections of different etiologies.40–42 This finding is consistent with our results. Importantly, we found that CHI3L1, IL-6, and TNF-α concentrations were highest at the beginning of the disease and decreased during the course of the infection. Elevated levels of cytokines in the CSF, particularly IL-6 and TNF-α, have been associated with more severe cases of viral meningitis and encephalitis in children. These cytokines are generally known to promote inflammation in CSN and contribute to poor neurological outcome.13,43,44 In our study a positive correlation with CHI3L1 was established among children with encephalitis. It is worth noting that there is a lack of studies that correlate mediator concentrations with the clinical picture in the pediatric population.
In the case of TBE, the exact mechanisms by which TBEV breach the BBB are not yet fully understood; however, different strategies have been postulated. For instance, the virus can enter the CNS by direct infection of the peripheral nerves and olfactory neurons through transcellular and paracellular entry into the endothelial cells of the BBB. The Trojan horse” mechanism and cytokine-mediated BBB breakdown also play vital roles in altering BBB.45,46 The results of our study suggest that infection with TBE causes the breakdown of the BBB, which is consistent with the results of similar studies. Increased levels of certain cytokines in the CSF have been observed in both animal and human studies, and their connection with BBB disruption of the BBB were established.45,47,48 TNF-α is a potent pro-inflammatory cytokine that directly affects the integrity of the BBB by increasing the permeability of endothelial cells and promoting the expression of adhesion molecules. TNF-α also stimulates the production of other cytokines and chemokines, which further contributes to BBB disruption.40 Second, IL-6 induces the expression of adhesion molecules in endothelial cells, thereby facilitating the migration of immune cells across the BBB. In addition, IL-6 can enhance the production of acute-phase proteins and is associated with poor neurological outcome.40,49 It is worth mentioning IFN-gamma - A cytokine that is primarily produced by activated T cells and natural killer (NK) cells. It plays a crucial role in antiviral defense but can also contribute to BBB disruption by inducing the expression of adhesion molecules on endothelial cells.50 In our study, we found that the albumin quotient decreased after treatment in cases of encephalitis. This suggests that the blood-brain barrier responds differently in encephalitis compared to meningitis. The differences in the index result from differences in the permeability of the BBB corresponding to the degree of damage, and provide insight into how the virus affects the BBB. Additionally, we found strong positive correlations between the albumin quotient and the levels of IL-4, IL-6, and TNF-α in the CSF. This indicates that as the albumin quotient increases, which reflects blood-brain barrier permeability, the concentrations of these inflammatory cytokines also rise. The correlation with IL-4 suggests a relationship with T-helper cell responses, while the associations with IL-6 and TNF-α highlight the involvement of pro-inflammatory pathways in the context of central nervous system inflammation. These findings may imply that increased blood-brain barrier permeability is associated with heightened inflammatory activity in the CSF, potentially reflecting the severity of the underlying condition.It is important to note that BBB disruption is independent of CD8+ T cells and occurs when CNS viremia is high.47
Therefore, the results of this study should be interpreted with caution. The examined group was relatively small, and in order to use these cytokines and chemokines in clinical practice, it would be necessary to establish age-correlated cutoff values. Although we excluded children with chronic diseases, the expression of chemokines/cytokines was linked to individual determinants, which requires further investigation. Moreover, obtaining CSF from healthy children is exceptionally restricted and these limitations are related to ethical reasons. Consequently, concentrations of the chemokines discussed in the CSF can only be compared with those in children who were admitted to the hospital with signs suggesting possible neuroinvasive infections that were subsequently excluded.
However, the advantage of our study is that it is a rare, clinically and microbiologically well-defined group of 22 children with TBE. We provide precise laboratory data. Concentrations of pro-/anti-inflammatory biomarkers were measured both in CSF and serum, and further adjusted to the albumin quotient. The study lacks control group of healthy individuals, but we used a within-subject design, where each patient served as their own control to assess changes over time. This approach allowed us to evaluate the temporal changes associated with disease progression and response to treatment, as observed in the significant reductions in CSF levels of certain markers post-treatment.
Conclusion
Our findings suggest that CHI3L1 is involved in the pathogenesis of TBE in children, however, causative relationships cannot be established in this observational study. We further point out that lower CHI3L1 CSF levels were observed in severe cases of TBE, supporting the potential utility of this protein as a marker for disease severity. Additionally, we identified significant interactions between CHI3L1 and other cytokines, specifically chemerin, IL-6, and TNF-α. Although the levels of chemerin remained stable over time, levels of CHI3L1, IL-6, and TNF-α decreased as clinical symptoms resolved. In summary, this study provides valuable insights into the immunopathological mechanisms of pediatric TBE and thus points to CHI3L1 and associated cytokines as the potential modulators of neuroinflammation and variability in the course of the disease.
Data Sharing Statement
Data available on reasonable request.
Ethical Approval
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Local Bioethics Committee of the Medical University of Bialystok (approval number APK.002.545.2023). All procedures performed in this study were undertaken as a part of routine clinical practice. Each patient was informed of the purpose of the study and written consent was obtained from their parents or caregivers.
Funding
This research was supported by the Medical University of Bialystok, Poland (to A.S. grant no. B.SUB.24.447). The funders played no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Disclosure
Professor Robert Flisiak reports grants, personal fees, non-financial support, Advisory from AbbVie, grants, personal fees, non-financial support, Advisory from Gilead, grants, personal fees, Advisory from Pfizer, grants from Roche, personal fees, non-financial support, Advisory from Novavax, Advisory from Novo Nordisk, outside the submitted work. The author reports no other conflicts of interest in this work.
References
1. Królasik A, Paradowska-Stankiewicz I. Meningitis and encephalitis in Poland in 2016. Przegl Epidemiol. 2018;72(3):293–301. doi:10.32394/pe.72.3.6
2. Bogovic P. Tick-borne encephalitis: a review of epidemiology, clinical characteristics, and management. WJCC. 2015;3(5):430. doi:10.12998/wjcc.v3.i5.430
3. Czupryna P, Grygorczuk S, Pancewicz S, et al. Evaluation of NSE and S100B in patients with tick-borne encephalitis. Brain Behav. 2018;8(12):e01160. doi:10.1002/brb3.1160
4. Bogovič P, Stupica D, Rojko T, et al. The long-term outcome of tick-borne encephalitis in Central Europe. Ticks Tick-Borne Dise. 2018;9(2):369–378. doi:10.1016/j.ttbdis.2017.12.001
5. Paradowska-Stankiewicz I, Pancer K, Poznańska A, et al. Tick-borne encephalitis epidemiology and surveillance in Poland, and comparison with selected European countries before and during the COVID-19 pandemic, 2008 to 2020. Eurosurveillance. 2023;28(18). doi:10.2807/1560-7917.ES.2023.28.18.2200452
6. COMMISSION IMPLEMENTING DECISION (EU) 2018/ 945 - of - on the communicable diseases and related special health issues to be covered by epidemiological surveillance as well as relevant case definitions.
7. Taba P, Schmutzhard E, Forsberg P, et al. EAN consensus review on prevention, diagnosis and management of tick-borne encephalitis. Eur J Neurol. 2017;24(10):1214–e61. doi:10.1111/ene.13356
8. Günther G, Haglund M, Lindquist L, Sköldenberg B, Forsgren M. Intrathecal IgM, IgA and IgG antibody response in tick-borne encephalitis. Long-term follow-up related to clinical course and outcome. Clin Diagn Virol. 1997;8(1):17–29. doi:10.1016/S0928-0197(97)00273-0
9. Stone ET, Pinto AK. T Cells in Tick-Borne Flavivirus Encephalitis: a Review of Current Paradigms in Protection and Disease Pathology. Viruses. 2023;15(4):958. doi:10.3390/v15040958
10. Yin R, Yang L, Hao Y, et al. Proteomic landscape subtype and clinical prognosis of patients with the cognitive impairment by Japanese encephalitis infection. J Neuroinflammation. 2022;19(1):77. doi:10.1186/s12974-022-02439-5
11. Růžek D, Vancová M, Tesařová M, Ahantarig A, Kopecký J, Grubhoffer L. Morphological changes in human neural cells following tick-borne encephalitis virus infection. J Gen Virol. 2009;90(7):1649–1658. doi:10.1099/vir.0.010058-0
12. Haglund M, Günther G. Tick-borne encephalitis—pathogenesis, clinical course and long-term follow-up. Vaccine. 2003; 21:S11–S18. doi:10.1016/S0264-410X(02)00811-3
13. Østergaard C, Johansen JS, Benfield T, Price PA, Lundgren JD. YKL-40 Is Elevated in Cerebrospinal Fluid from Patients with Purulent Meningitis. Clin Vaccine Immunol. 2002;9(3):598–604. doi:10.1128/CDLI.9.3.598-604.2002
14. Chen J, Ding Y, Zheng D, et al. Elevation of YKL-40 in the CSF of Anti-NMDAR Encephalitis Patients Is Associated With Poor Prognosis. Front Neurol. 2018;9:727. doi:10.3389/fneur.2018.00727
15. Dorcet G, Benaiteau M, Pariente J, et al. Cerebrospinal fluid YKL‐40 level evolution is associated with autoimmune encephalitis remission. Clin & Trans Imm. 2023;12(3):e1439. doi:10.1002/cti2.1439
16. Baldacci F, Lista S, Palermo G, Giorgi FS, Vergallo A, Hampel H. The neuroinflammatory biomarker YKL-40 for neurodegenerative diseases: advances in development. Expert Revi Proteo. 2019;16(7):593–600. doi:10.1080/14789450.2019.1628643
17. Bardina SV, Lim JK. The role of chemokines in the pathogenesis of neurotropic flaviviruses. Immunol Res. 2012;54(1–3):121–132. doi:10.1007/s12026-012-8333-3
18. Grygorczuk S, Świerzbińska R, Kondrusik M, et al. The intrathecal expression and pathogenetic role of Th17 cytokines and CXCR2-binding chemokines in tick-borne encephalitis. J Neuroinflammation. 2018;15(1):115. doi:10.1186/s12974-018-1138-0
19. Zajkowska J, Moniuszko-Malinowska A, Pancewicz S, et al. Evaluation of CXCL10, CXCL11, CXCL12 and CXCL13 chemokines in serum and cerebrospinal fluid in patients with tick borne encephalitis (TBE). Adv Med Sci. 2011;56(2):311–317. doi:10.2478/v10039-011-0033-z
20. Guziejko K, Czupryna P, Pancewicz S, et al. Analysis of CCL-4, CCL-17, CCL-20 and IL-8 concentrations in the serum of patients with tick-borne encephalitis and anaplasmosis. Cytokine. 2020;125:154852. doi:10.1016/j.cyto.2019.154852
21. Bogovic P, Logar M, Avsic-Zupanc T, Strle F, Lotric-Furlan S. Quantitative Evaluation of the Severity of Acute Illness in Adult Patients with Tick-Borne Encephalitis. Biomed Res Int. 2014;2014:1–5. doi:10.1155/2014/841027
22. Reiber H. External quality assessment in clinical neurochemistry: survey of analysis for cerebrospinal fluid (CSF) proteins based on CSF/serum quotients. Clin Chem. 1995;41(2):256–263. doi:10.1093/clinchem/41.2.256
23. Ben-Shachar M, Lüdecke D, Makowski D. effectsize: estimation of Effect Size Indices and Standardized Parameters. JOSS. 2020;5(56):2815. doi:10.21105/joss.02815
24. Chan Y, Westbrook R, Holmes N. Protecting the Rescorla-Wagner (1972) theory: a reply to Spicer et al. J Experimental Psyc. 2021;47:211–215. doi:10.1037/xan0000271
25. Makowski D, Catarino R, Chen M, et al. Synthesising results of meta-analyses to inform policy: a comparison of fast-track methods. Environ Evid. 2023;12(1):16. doi:10.1186/s13750-023-00309-y
26. Patil I. Visualizations with statistical details: the “ggstatsplot” approach. JOSS. 2021;6(61):3167. doi:10.21105/joss.03167
27. Sjoberg DD, Whiting K, Curry M, Lavery JA, Larmarange J. Reproducible Summary Tables with the gtsummary Package. R J. 2021;13(1):570. doi:10.32614/RJ-2021-053
28. Thiele C, Hirschfeld G. cutpointr: improved Estimation and Validation of Optimal Cutpoints in R. J Stat Soft. 2021;98(11):1–27. doi:10.18637/jss.v098.i11
29. Wickham H, Averick M, Bryan J, et al. Welcome to the Tidyverse. JOSS. 2019;4(43):1686. doi:10.21105/joss.01686
30. Wickham H, François R Dplyr: a Grammar of Data Manipulation; 2014.
31. Shao R. YKL-40 acts as an angiogenic factor to promote tumor angiogenesis. Front Physiol. 2013;4. doi:10.3389/fphys.2013.00122
32. Wang K, Deubel V. Mice with Different Susceptibility to Japanese Encephalitis Virus Infection Show Selective Neutralizing Antibody Response and Myeloid Cell Infectivity. PLoS One. 2011;6(9):e2244744. doi:10.1371/journal.pone.0024744
33. Denizot M, Neal JW, Gasque P. Encephalitis due to emerging viruses: CNS innate immunity and potential therapeutic targets. J Infect. 2012;65(1):1–16. doi:10.1016/j.jinf.2012.03.019
34. Zhang W, Zhou X, Yin J, et al. YKL-40 as a novel biomarker related to white matter damage and cognitive impairment in patients with cerebral small vessel disease. Brain Res. 2023;1807:148318. doi:10.1016/j.brainres.2023.148318
35. Li J, Lin J, Pan Y, et al. Interleukin-6 and YKL-40 predicted recurrent stroke after ischemic stroke or TIA: analysis of 6 inflammation biomarkers in a prospective cohort study. J Neuroinflammation. 2022;19(1):131. doi:10.1186/s12974-022-02467-1
36. YKL-40 as a novel diagnostic biomarker in Toxoplasmosis. JPTCP. 2022;29(2). doi:10.47750/jptcp.2022.932
37. Muszyński P, Groblewska M, Kulczyńska-Przybik A, Kułakowska A, Mroczko B. YKL-40 as a Potential Biomarker and a Possible Target in Therapeutic Strategies of Alzheimer’s Disease. CN. 2017;15(6). doi:10.2174/1570159X15666170208124324
38. Kim KY, Ahn Y, Kim DY, Kim HS, Kim DS. Elevated serum YKL-40 levels in patients with Kawasaki disease. Biomarkers. 2017;22(3–4):326–330. doi:10.1080/1354750X.2016.1265000
39. Czupryna P, Kulczyńka-Przybik A, Mroczko B, et al. Assessment of the YKL-40 concentration in patients with tick-borne encephalitis. Ticks Tick-Borne Dise. 2022;13(2):101895. doi:10.1016/j.ttbdis.2022.101895
40. Fowler Å, Ygberg S, Bogdanovic G, Wickström R. Biomarkers in Cerebrospinal Fluid of Children With Tick-borne Encephalitis: association With Long-term Outcome. Pediatr Infect Dis J. 2016;35(9):961–966. doi:10.1097/INF.0000000000001210
41. Sato M, Hosoya M, Honzumi K, et al. Cytokine and Cellular Inflammatory Sequence in Enteroviral Meningitis. Pediatrics. 2003;112(5):1103–1107. doi:10.1542/peds.112.5.1103
42. Ichiyama T, Ito Y, Kubota M, Yamazaki T, Nakamura K, Furukawa S. Serum and cerebrospinal fluid levels of cytokines in acute encephalopathy associated with human herpesvirus-6 infection. Brain Dev. 2009;31(10):731–738. doi:10.1016/j.braindev.2008.11.005
43. Kamei S, Taira N, Ishihara M, et al. Prognostic value of cerebrospinal fluid cytokine changes in herpes simplex virus encephalitis. Cytokine. 2009;46(2):187–193. doi:10.1016/j.cyto.2009.01.004
44. Hasegawa S, Matsushige T, Inoue H, Shirabe K, Fukano R, Ichiyama T. Serum and cerebrospinal fluid cytokine profile of patients with 2009 pandemic H1N1 influenza virus-associated encephalopathy. Cytokine. 2011;54(2):167–172. doi:10.1016/j.cyto.2011.01.006
45. Dörrbecker B, Dobler G, Spiegel M, Hufert FT. Tick-borne encephalitis virus and the immune response of the mammalian host. Travel Med Infectious Dis. 2010;8(4):213–222. doi:10.1016/j.tmaid.2010.05.010
46. Růžek D, Dobler G, Mantke OD. Tick-borne encephalitis: pathogenesis and clinical implications. Travel Med Infectious Dis. 2010;8(4):223–232. doi:10.1016/j.tmaid.2010.06.004
47. Růžek D, Salát J, Singh SK, Kopecký J. Breakdown of the Blood-Brain Barrier during Tick-Borne Encephalitis in Mice Is Not Dependent on CD8+ T-Cells. PLoS One. 2011;6(5):e20472. doi:10.1371/journal.pone.0020472
48. Atrasheuskaya AV, Fredeking TM, Ignatyev GM. Changes in immune parameters and their correction in human cases of tick-borne encephalitis. Clin Exp Immunol. 2003;131(1):148–154. doi:10.1046/j.1365-2249.2003.02050.x
49. Palus M, Formanová P, Salát J, Žampachová E, Elsterová J, Růžek D. Analysis of serum levels of cytokines, chemokines, growth factors, and monoamine neurotransmitters in patients with tick-borne encephalitis: identification of novel inflammatory markers with implications for pathogenesis: serum Inflammatory Markers in TBE. J Med Virol. 2015;87(5):885–892. doi:10.1002/jmv.24140
50. Zidovec-Lepej S, Vilibic-Cavlek T, Ilic M, et al. Quantification of Antiviral Cytokines in Serum, Cerebrospinal Fluid and Urine of Patients with Tick-Borne Encephalitis in Croatia. Vaccines. 2022;10(11):1825. doi:10.3390/vaccines10111825
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





