Back to Journals » Clinical Ophthalmology » Volume 18
The Toronto Tele-Retinal Screening Program for the Elderly in Long-Term Care: A Pilot Project
Authors Lim M, Felfeli T , Mangubat W, Moghimi H, Grinton M, Brent MH
Received 12 August 2024
Accepted for publication 7 December 2024
Published 20 December 2024 Volume 2024:18 Pages 3881—3892
DOI https://doi.org/10.2147/OPTH.S491154
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Michelle Lim,1 Tina Felfeli,2,3 Winnie Mangubat,4 Hamid Moghimi,4 Michael Grinton,5 Michael H Brent1,2,6
1Faculty of Medicine, University of Toronto, Toronto, ON, Canada; 2Department of Ophthalmology and Vision Sciences, University of Toronto, Toronto, ON, Canada; 3Institute of Health Policy, Management and Evaluation, Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada; 4South Riverdale Community Health Centre, Toronto, ON, Canada; 5Department of Ophthalmology, Sunderland Eye Infirmary, Sunderland, UK; 6Department of Ophthalmology, Toronto Western Hospital, University Health Network, Toronto, ON, Canada
Correspondence: Michael H Brent, Email [email protected]
Objective: To report the results and feasibility of a pilot expansion of the Toronto Tele-Retinal Screening Program in an elderly long-term care home.
Methods: Long term care patients with Type II diabetes mellitus (DM) were screened between April 1, 2022, and July 1, 2022. Demographic and health data were collected through surveys.
Results: A total of 28 patients were screened, with 85.7% successfully undergoing retinal imaging. Among imaged patients, 8.3% (2/24) required urgent follow-up. Pathologies identified included uncontrolled glaucoma (4.1%, 1/24), non-proliferative diabetic retinopathy (8.3%, 2/24), and age-related macular degeneration (45.8%, 11/24). The handheld camera successfully screened 60% (3/5) of patients with mobility issues. Overall, 90% (17/19) of patients rated their experience as either “brilliant” or “really good”.
Discussion: This pilot project demonstrated the necessity for routine eye care in the elderly and the potential for widespread implementation of teleophthalmology in long-term care facilities. With only 14.3% (4/28) of patients unable to be imaged, this program offers a feasible, patient-friendly alternative to in-clinic screening. Future policies and practices in teleophthalmology should consider the unique needs of long-term care residents and the potential for reducing healthcare disparities through such a program.
Keywords: teleophthalmology, diabetes, retinopathy, elderly care, telemedicine, healthcare accessibility
Introduction
Diabetic retinopathy (DR) poses a significant threat to vision and affects approximately one-third of patients with diabetes.1 Furthermore, sight-threatening retinopathy develops in one-third of patients who develop DR. Globally, DR stands as the primary cause of blindness among working-age adults.2 Elderly individuals residing in long-term care facilities face an elevated risk of various sight-threatening ocular conditions including DR, age-related macular degeneration (AMD), retinal occlusive diseases, and primary open-angle glaucoma.3 These conditions often manifest asymptomatically, leading to missed opportunities for timely intervention and irreversible vision loss.4 Recognizing this, Diabetes Canada recommends annual retinopathy screening for individuals with type 2 diabetes.5
Despite these recommendations, the elderly face obstacles to timely and regular ophthalmic treatment such as transportation to eye clinics and financial barriers to ophthalmic imaging. These barriers are particularly burdensome considering the prevalence of comorbidities, reliance on pension plans, and the financial demands of long-term care homes. However, emerging evidence suggests that early preventative screening for ocular diseases not only mitigates vision loss but also yields cost-effective benefits for healthcare systems.6,7 Moreover, vision loss not only reduces quality of life but also correlates with double the risk of falls, earlier placement in nursing homes, social decline, and depression,8 highlighting the importance of routine screening in elderly patients with diabetes.
Teleretina programs are the most widely established branch of teleophthalmology, specifically focusing on the remote assessment of retinal conditions, such as diabetic retinopathy, and are increasingly used in diabetic screening.9 Teleretinal screening programs demonstrate a diagnostic accuracy comparable to traditional face-to-face examinations for the detection of DR and AMD.10 Over the past decade, the Toronto Tele-Retinal Screening Program has been successfully implemented to circumvent the low rate of DR screening in patients.11 We have previously demonstrated that our program can be effective in an urban, culturally diverse, and socioeconomically disadvantaged population.12
Applications of teleophthalmology screening programs for the elderly have been explored to date and are essential for identifying unknown ophthalmological diseases that require specialized management.13 Members of this population often face intersectional vulnerabilities, including significant language barriers. Such barriers can impede navigation of the healthcare system. The Toronto Tele-Retina Screening Program addresses these challenges by providing language support to facilitate improved communication. Additionally, our program allows for remote consultations, reducing travel time and allowing screenings in familiar community settings. Beyond the immediate benefit of disease identification, teleretina programs hold significant potential to become established as the standard of care for vulnerable populations in long-term care settings.14 By facilitating timely access to essential eye screenings, these programs not only improve early detection and intervention but also contribute to a more efficient healthcare system and enhance patient outcomes.14,15
This study aimed to assess the feasibility and outcomes of implementing the Toronto Tele-Retinal Screening Program in elderly patients with type 2 diabetes in a long-term care setting. Our findings will inform tailored DR management strategies for this vulnerable patient cohort, serving as proof-of-concept for integrated care delivery to individuals with diabetes and complex care needs.
Materials and Methods
This prospective pilot project aimed to implement the Toronto Tele-Retinal Screening Program for elderly patients with type II diabetes mellitus residing in the Mon Sheong Home for the Aged. Patients were referred to the Toronto Tele-Retinal Screening Program for screening between April 1, 2022, and July 1, 2022. The inclusion criteria for the tele-retinal screening program were adults aged ≥18 years with type II diabetes who had not undergone an eye examination within the past year or could not recall their last eye exam, had no current eye care provider involved in their ocular health, and had provided written informed consent for study participation. We specifically selected patients who had not undergone an eye examination within the past year to ensure that we could identify undiagnosed ocular conditions. Notably, the patient population primarily consisted of Chinese individuals with limited English proficiency. This project was approved by the University Health Network Review Committee and Clinical Trials Ontario (Project ID#1514) and adhered to the ethical principles outlined in the Declaration of Helsinki.
Data Collection
Prior to screening, demographic and health data were collected, including age, sex, marital status, ethnicity, native language, health insurance status, education, diabetes-related information, ocular history, mobility status, and satisfaction with the program. Surveys were administered at the beginning of the screening session to gather the health history and mobility information (Appendix Figure 1). For patients with cognitive impairment, inquiries were directed to family members or nurses, when available.
Screening Procedures
Team members from the Toronto Tele-Retinal Screening Program visited the Mon Sheong Home for the Aged for over three days to conduct screenings using both tabletop and portable handheld cameras. Certified Cantonese translators facilitated communication during the screening process. Visual acuity was assessed using the Snellen chart with habitual correction and/or pinhole, and intraocular pressure was measured using a Tono-pen (Reichert Tono-Pen XL Applanation Tonometer). Prior to image capture, all patients received 1.0% tropicamide. Monoscopic color fundus photographs and optical coherence tomography (OCT) scans were obtained using the Clarion iFusion tabletop camera (Clarion Medical Technologies, Cambridge, Canada) and the Volk Pictor Prestige portable fundus camera (Volk Optical, Ohio). Handheld images were captured by the medical student, ophthalmology clinical fellow, and ophthalmology resident (M.L., M.G., and T.F)., whereas the tabletop camera was operated by two trained employees of the South Riverdale Community Health Centre experienced with its use (W.M. and H.M). Bedridden patients who were unable to sit independently for tabletop camera imaging underwent screening solely using the handheld camera. Imaging specifications included a scan speed of 50,000 A-scans per second, lateral resolution of 20 µm, and in-depth resolution of 6 µm. Red-free images were acquired when color fundus photographs did not provide sufficient clarity.
Data Processing and Analysis
All data were uploaded and stored using Store Forward technology provided by Ontario Health (OTN) and subsequently graded by an experienced ophthalmologist in the medical retina fellowship training program under the supervision of a senior retina specialist staff (M.H.B.) at the Department of Ophthalmology, Toronto Western Hospital. Following each review of the images and clinical data, a summary report was generated which included the following graded data: corrected visual acuity, intraocular pressure, presence of DR, classification of DR into non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR), presence of macular edema, and presence of any other ophthalmic pathology. Screening outcomes were categorized into four groups: “No pathology identified”, “Non-urgent pathology identified”, “Urgent pathology identified”, and “Insufficient image quality”. Subsequent actions were recommended based on screening results (Figure 1).
Comparison of Imaging Devices
The selection of the Volk Pictor Prestige portable handheld fundus camera and the Clarion iFusion tabletop camera was based on their availability and previously demonstrated diagnostic sensitivity and specificity.15 Comparative assessments were made regarding the resolution, clarity of the retinal anatomy (eg, retinal vessels), and absence of glare or artifacts. Portability was compared by assessing the ability of each device to screen for bedbound patients and their ease of transport. Diagnostic accuracy was evaluated through blinded diagnosis by a retina specialist (M.G)., with findings presented in tabular format for comparison.
Statistical Analysis
Descriptive statistics were used to report all collected outcomes for the entire cohort and subcohorts of patients with and without DR. All parametric data are presented as mean and standard deviation (SD). All statistical analyses were performed using Microsoft Excel Version 15.31 (Microsoft Corporation, Redmond, WA, USA). Questions regarding satisfaction with the Toronto Tele-Retinal Screening Program were completed using Likert scales.16
Results
Patient Demographics
A total of 28 elderly patients with type II diabetes mellitus were screened by the Toronto Tele-Retinal Screening Program pilot project during the study period. The patients who underwent DR screening were on average 86.5 years old (Table 1 and Appendix Table A.1). Notably, only 4% (1/28) of the patients spoke English, and only 16.67% (3/18) had college education. Common medical conditions included hypertension (93%, 26/28), osteoporosis (57%, 16/28), previous stroke (36%, 10/28), and arthritis (32%, 9/28). For patients who were unable to recall their health history, collateral information was gathered from the nurses and family members.
 |
Table 1 Aggregate Data on Baseline Patient Characteristics and Screening Results |
Most patients were either unable to recall their last eye exam (33%, 5/15), had their last eye exam 3 years ago (33%, 5/15), had their last eye exam more than 10 years ago (20%, 3/15), or had never had an eye exam (13%, 2/15). There were 93 residents at the time of screening; therefore, 30.1% (28/93) of patients did not adhere to the recommended screening guidelines. Most patients reported no (47%, 9/19) or some (42%, 8/19) difficulty seeing. Half of the patients had a fall in the last year (50%, 14/28) and 35% (10/28) were fully dependent for mobility. One-quarter of the patients (12/28) indicated difficulty rising from bed and independently sitting on a chair, whereas 32% (9/28) were unable to perform these activities independently.
Screening Results
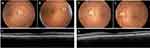
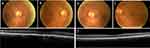
Four patients (14%) did not undergo fundus imaging due to severe cognitive impairment, dense cataracts, or refusal (Appendix Table A.1). Of the patients successfully screened, the majority (79%, 19/24) had no urgent pathology identified and required repeat screening within one year (Figure 2). Two patients (8%) were advised to undergo screening at an increased frequency as they had non-urgent pathology identified (Figure 3), and another two had urgent pathology identified (Figure 4). One patient (4%) had insufficient image quality and required follow-up to undergo in-clinic screening.
Pathologies Identified
Of the two patients with urgent pathology identified, one had a history of glaucoma and had an IOP of 32 mmHg. The other patient had a macular hemorrhage with pigment epithelial detachment and subretinal fluid in the right eye, likely indicating wet AMD and moderate NPDR (Figure 4). Incidental findings included dense cataracts, disc cupping, drusen (Figure 5), epiretinal membranes, lamellar holes, and orbital fat prolapse (Appendix Figure 2).
Survey Results
Eight patients (29%) were unable to answer the questionnaire because of cognitive impairment (Appendix Table A.2). Most patients (81%, 21/26) were able to attend a hospital appointment if needed and had either a family member or a public trustee who could take them to medical appointments (Table 2). Five patients (19%) did not have anyone to take them to medical appointments. Of these five patients, three (60%) were willing to attend a hospital appointment if transportation was provided. A total of four patients (20%) were unwilling to attend a hospital appointment due to a lack of interest in our program (two patients) or no reason provided (two patients). Overall, 89% (17/19) of patients rated their screening experience as either “brilliant” (68%, 13/19) or “really good” (21%, 4/19). One patient could not rate their experience, while another patient rated their experience as “not very good” because of the unfulfilled expectations of our screening program. Upon further questioning, the patient revealed that they expected new glass prescriptions and felt overwhelmed by the screening steps. Regarding the likelihood of returning for screening, 84% (16/19) of the patients were either very likely (74%, 14/19) or likely (10%, 2/19) to return to the screening program.
 |
Table 2 Aggregate Data for Survey Results |
Comparison Between Handheld and Tabletop Devices
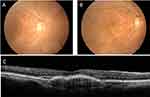
Fundus images from the two modalities were retrospectively reviewed and diagnosed (Appendix Table A.3). Five patients (20.8%, 5/24) were unable to undergo tabletop imaging because of mobility issues. Screening with the handheld camera was successful in 60% (3/5) of the patients. The diagnoses missed by the handheld camera and identified by the tabletop were dry AMD (12.5%, 3/24) and mild NPDR (8.3%, 2/24). The handheld camera’s ability to capture images of wheelchair-bound patients was proven useful when the tabletop image could not capture the disc of one patient because of mobility issues (Appendix Figure 3). The main drawback of the handheld camera was the glare on the inferior portion of the images (Appendix Figure 4), which persisted in images of 7/24 (29.2%) patients. The advantage of the built-in OCT imager of the tabletop camera was demonstrated when one patient’s equivocal macular edema on fundus imaging was confirmed by OCT (Figure 6).
Discussion
This pilot study aimed to assess the feasibility and clinical impact of implementing the Toronto Tele-Retinal Screening Program in elderly patients residing in a long-term care facility. Of the 93 residents, 28 (30.1%) underwent retinopathy screening, indicating that a significant proportion did not adhere to Diabetes Canada’s recommended annual screening.5 This adherence rate is comparable to that of the general Ontario diabetic population, where approximately 35% have not undergone screening within a two-year period.16 A substantial proportion (35%) of participants relying on full mobility assistance highlighted the necessity of extending such programs to nursing homes.
The intersection of cognitive impairment and vision loss doubles the risk of falls and dependence on activities of daily living.17 Therefore, expanding teleophthalmology to long-term care settings circumvents transportation challenges and facilitates direct healthcare delivery to patients. Notably, our program demonstrated a high success rate (85.7%) in obtaining retinal images, making it a viable alternative to traditional clinical screening. This is in comparison to diabetic retinopathy screening programs in Liverpool and Danish nursing homes, which were unable to screen 13–20% of participants due to poor cooperation.18,19 Importantly, the screening programs reported participant numbers ranging from 371 to 2427, therefore demonstrating the potential scalability of a teleretina program. Cognitive decline itself may also act as a barrier to regular healthcare. Previous studies have demonstrated that patients with dementia have higher levels of untreated dental decay than those without dementia, demonstrating the critical role of cognitive function in healthcare.20,21 The Toronto Tele-Retinal Screening Program may mitigate cognitive decline as a screening barrier.
Beyond retinal pathologies, our screening program identified dense cataracts as well as various sight-threatening conditions including severe glaucoma and wet AMD, both of which would likely result in irreversible blindness if left untreated. Previous studies on eye examinations in nursing homes found that 35% of residents had sight-threatening eye diseases18 and 36% required referral to an ophthalmologist.19 Evidently, ocular pathologies are present in the elderly population; a mobile screening program brought to homes can help with the early identification and treatment of these pathologies.
Transportation barriers remain a significant obstacle to accessing healthcare.22,23 In our study, most patients were willing to attend hospital appointments if transportation was provided, suggesting that this barrier could be eliminated. It is important to note that, while most patients expressed readiness to attend hospital appointments with provided transportation, a subset (14%) remained unwilling without a specified reason. This question was central to our screening program, as it is currently not feasible to provide laser or intravitreal injections at the bedside because of the need for specialized equipment, a sterile environment, transport of refrigerated medications, and lack of ophthalmologists available to visit nursing homes. Thus, the success of this screening program requires patients to be willing to visit the hospital for treatment if they have pathology, similar to other retinal screening programs.18,24–27
Additionally, inconsistent responses from participants, possibly attributable to cognitive impairment and/or language barriers, demonstrate the complexity of communication in this setting. For example, one patient informed us of no interest in the screening program but indicated that they would be very likely to return for regular screening. Cognitive impairment was addressed by collecting collateral information from family members and enlisting help of nursing staff who had established long-term relationships with the patient. This approach not only provided valuable insights but also offered a sense of familiarity that helped guide the patient during the process. For patients with language barriers, we were able to successfully explain our screening process and questionnaire using a certified translator. As a result, these patients were able to navigate the screening process effectively. However, for less common languages such as Shanghainese and Fukien, we encountered challenges in accurate communication among participants. This lack of communication is an important issue to highlight as it has been previously shown that, in Chinese American immigrants, English proficiency as well as health literacy are directly associated with health status.28 Importantly, one patient was dissatisfied with our program because she expected new glasses prescriptions and felt overwhelmed by the screening process. As we are now aware of this, future screening sessions should clearly indicate goals of the session and what patients can expect at a slower pace, especially for those with language or cognitive barriers. Finally, although teleophthalmology enhances access to care, its equitable distribution remains unclear.29 Investment in such technology in resource-limited settings, which are often communities of racial and ethnic minorities, could promote equitable eye-care access among vulnerable populations.
Unlike a tabletop camera, a handheld camera can be disassembled in a compact briefcase, allowing for easy transport between facilities. As the camera is not attached to a table, it can be easily maneuvered to view the retina of bedridden patients who cannot sit for an exam (Appendix Figure 3). The importance of this point-and-shoot nature of the handheld camera was demonstrated for one patient, whose disc could not be imaged with the tabletop but was successfully imaged with the handheld camera to rule out diabetic retinopathy (Appendix Table A.3). However, handheld cameras also have several limitations. First, the resolution and clarity of the images captured by the tabletop camera outweighed those of the handheld camera (Appendix Table A.3). Consequently, images from tabletop cameras are preferentially used for screening. Glare at the bottom of the fundus photos was also a common occurrence, obscuring the inferior retina (Appendix Figure 4). The tabletop imager provided OCT scans that supplemented the diagnosis. For example, tabletop images from one patient showed an equivocal edema in the right eye. The presence of the edema was confirmed with OCT (Figure 6). Interestingly, a recent study demonstrated that Volk Pictor Plus achieved an image quality, gradeability, and diagnostic specificity comparable to those of the tabletop Zeiss Visucam.14 For example, the diagnostic specificity between the Pictor and Visucam were observed to be similar (Zeiss, mean 82.0%; Pictor, mean 83.0%). In this study, a notable proportion (41.6%, 10/24) of diagnoses between the tabletop and handheld images were identical despite insufficient user experience. Thus, it is reasonable to believe that with more experience, the handheld camera may be an excellent option in resource-limited settings or with a high proportion of bedbound patients who would not have been screened otherwise.
Effective implementation of teleophthalmology requires a workforce of adequately trained providers, the availability of the relevant technology, and the logistics of scaling such a program. The establishment of comprehensive training programs for handheld cameras would be essential to the success of the program. As demonstrated by a village screening camp in Nepal, ophthalmic technicians can be trained to carry out large-scale screening programs.30 The initial investment in high-quality imaging devices can be a barrier in itself for low-resource settings that would benefit the most from a teleophthalmology program. Alternatives such as smartphone-based imaging systems (eg Remidio Fundus) have shown high accuracy in detecting diabetic retinopathy and can be a less expensive alternative.31 Lastly, when considering the scalability of these programs, logistical factors such as patient referral processes, data management systems, and follow-up care coordination must be addressed. Establishing partnerships with local healthcare facilities can facilitate a streamlined administration pathway, allowing for the smooth transition of patients needing in-person consultations after remote screenings.
The limitations of this study include the small sample size and homogeneity of the patient population. Therefore, this population may not adequately represent the diverse characteristics and needs of individuals in long-term care settings. This lack of diversity can obscure important variations in health outcomes and healthcare accessibility that might be observed in a more varied group, limiting the applicability of the results to the broader long-term care population. Moreover, data inconsistencies and missing survey responses underscore the challenges inherent to conducting research in long-term care settings. Inconsistent answers may be due to factors beyond our control, such as cognitive impairment or dementia, or those within our control, such as inadequate language translation. Future studies should aim for larger, more diverse nursing homes across Ontario that employ multiple translators in various languages identified beforehand, to enhance data accuracy and reliability.
Conclusion
In conclusion, the pilot expansion of the Toronto Tele-Retinal Screening Program identified the need for routine eye care in the elderly population in long-term care homes. It revealed the lack of regular screening despite recommendations from Diabetes Canada, as well as multiple challenges contributing to this, such as language and mobility barriers. Our study demonstrated that the implementation of teleophthalmology screening for the elderly in long-term care homes mitigates common barriers to eye screening.
Abbreviations
AMD, age-related macular degeneration; DM, diabetes mellitus; DR, diabetic retinopathy; HCP, healthcare provider; IOP, intraocular pressure; NPDR, non-proliferative diabetic retinopathy; OCT, optical coherence tomography; OHIP, Ontario Health Insurance Plan; OTN, Ontario Telehealth Network; PDR, proliferative diabetic retinopathy.
Disclosure
The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This work was supported by Diabetes Action Canada. This paper has been uploaded to JMIR Publications as a preprint:https://preprints.jmir.org/preprint/49188. The Authors declare that there is no conflict of interest.
References
1. Saaddine JB, Honeycutt AA, Narayan KMV, Zhang X, Klein R, Boyle JP. Projection of diabetic retinopathy and other major eye diseases among people with diabetes mellitus: United States, 2005-2050. Arch Ophthalmol. 2008;126(12):1740–1747. doi:10.1001/archopht.126.12.1740
2. Ting DSW, Cheung GCM, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44(4):260–277. doi:10.1111/ceo.12696
3. Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221–e1234. doi:10.1016/S2214-109X(17)30393-5
4. Eichenbaum JW. Geriatric vision loss due to cataracts, macular degeneration, and glaucoma. Mount Sinai J Med. 2012;79(2):276–294. doi:10.1002/msj.21303
5. Altomare F, Kherani A, Lovshin J. Retinopathy. Can J Diabetes. 2018;42(Suppl 1):S210–S216. doi:10.1016/j.jcjd.2017.10.027
6. Avidor D, Loewenstein A, Waisbourd M, Nutman A. Cost-effectiveness of diabetic retinopathy screening programs using telemedicine: a systematic review. Cost Eff Resour Alloc. 2020;18:16. doi:10.1186/s12962-020-00211-1
7. Stanimirovic A, Francis T, Shahid N, et al. Tele-retina screening of diabetic retinopathy among at-risk populations: an economic analysis. Can J Ophthalmol. 2020;55:8–13. doi:10.1016/j.jcjo.2019.06.008
8. Green C, Goodfellow J, Kubie J. Eye care in the elderly. Aust Fam Physician. 2014;43(7):447–450.
9. Surendran TS, Raman R. Teleophthalmology in diabetic retinopathy. J Diabetes Sci Technol. 2014;8(2):262–266. doi:10.1177/1932296814522806
10. Mehraban Far P, Tai F, Ogunbameru A, et al. Diagnostic accuracy of teleretinal screening for detection of diabetic retinopathy and age-related macular degeneration: a systematic review and meta-analysis. BMJ Open Ophthalmol. 2022;7(1):e000915. doi:10.1136/bmjophth-2021-000915
11. Felfeli T, Alon R, Merritt R, Brent MH. Toronto tele-retinal screening program for detection of diabetic retinopathy and macular edema. Can J Ophthalmol. 2019;54(2):203–211. doi:10.1016/j.jcjo.2018.07.004
12. Cao J, Felfeli T, Merritt R, Brent MH. Sociodemographics associated with risk of diabetic retinopathy detected by tele-ophthalmology: 5-year results of the Toronto tele-retinal screening program. Can J Diabetes. 2021;46(1):26–31. doi:10.1016/j.jcjd.2021.05.001
13. Boureau AS, Masse H, Chapelet G, et al. Tele-ophthalmology for screening for eye diseases in older patients with cognitive complaints. J Telemed Telecare. 2021;27(8):493–500. doi:10.1177/1357633X19893883
14. Daskivich LP, Vasquez C, Martinez CJ, Tseng CH, Mangione CM. Implementation and evaluation of a large-scale teleretinal diabetic retinopathy screening program in the Los Angeles county department of health services. JAMA Intern Med. 2017;177(5):642–649. doi:10.1001/jamainternmed.2017.0204 PMID: 28346590; PMCID: PMC5818774.
15. Das S, Kuht HJ, De Silva I, et al. Feasibility and clinical utility of handheld fundus cameras for retinal imaging. Eye. 2023;37(2):274–279. doi:10.1038/s41433-021-01926-y
16. Likert R. A technique for the measurement of attitudes. Arch Psychol. 1932;140:5–55.
17. Jacobs JM, Hammerman-Rozenberg R, Maaravi Y, Cohen A, Stessman J. The impact of visual impairment on health, function and mortality. Aging Clin Exp Res. 2005;17(4):281–286. doi:10.1007/BF03324611
18. Anderson S, Broadbent DM, Swain JYS, Vora JP, Harding SP. Ambulatory photographic screening for diabetic retinopathy in nursing homes. Eye. 2003;17(6):711–716. doi:10.1038/sj.eye.6700487
19. Jensen H, Tubæk G. Elderly people need an eye examination before entering nursing homes. Danish Med J. 2017;64(2):A5325.
20. Thomson WM, Smith MB, Ferguson CA, Kerse NM, Peri K, Gribben B. Oral status, cognitive function and dependency among New Zealand nursing home residents. Gerodontology. 2018;35(3):185–191. doi:10.1111/ger.12337
21. Gopalakrishnan A, Kahu E, Jones L, Brunton P. Access and barriers to oral health care for dependent elderly people living in rest homes. Gerodontology. 2019;36(2):149–155. doi:10.1111/ger.12392
22. Rittner B, Kirk AB. Health care and public transportation use by poor and frail elderly people. Soc Work. 1995;40(3):365–373. doi:10.1093/sw/40.3.365
23. Fitzpatrick AL, Powe NR, Cooper LS, Ives DG, Robbins JA, Enright E. Barriers to health care access among the elderly and who perceives them. Am J Public Health. 2004;94(10):1788–1794. doi:10.2105/AJPH.94.10.1788
24. Boucher MC, Desroches G, Garcia-Salinas R, et al. Teleophthalmology screening for diabetic retinopathy through mobile imaging units within Canada. Can J Ophthalmol. 2008;43(6):658–668. doi:10.3129/i08-120
25. Nathoo N, Ng M, Rudnisky CJ, Tennant MTS. The prevalence of diabetic retinopathy as identified by teleophthalmology in rural Alberta. Can J Ophthalmol. 2010;45(1):28–32. doi:10.3129/i09-220
26. Arora S, Kurji AK, Tennant MTS. Dismantling sociocultural barriers to eye care with tele-ophthalmology: lessons from an Alberta Cree community. Clin Investig Med. 2013;36(2):E57–E63. doi:10.25011/cim.v36i2.19567
27. Kanjee R, Dookeran RI, Mathen MK, Stockl FA, Leicht R. Six-year prevalence and incidence of diabetic retinopathy and cost-effectiveness of tele-ophthalmology in Manitoba. Can J Ophthalmol. 2016;51(6):467–470. doi:10.1016/j.jcjo.2016.05.002
28. Tsoh JY, Sentell T, Gildengorin G, et al. Healthcare communication barriers and self-rated health in older Chinese American immigrants. J Community Health. 2016;41(4):741–752. doi:10.1007/s10900-015-0148-4
29. Scanzera AC, Kim SJ, Paul Chan RV. Teleophthalmology and the digital divide: inequities highlighted by the COVID-19 pandemic. Eye. 2021;35(6):1529–1531. doi:10.1038/s41433-020-01323-x
30. Collon S, Chang D, Tabin G, et al. Utility and feasibility of teleophthalmology using a smartphone-based ophthalmic camera in screening camps in Nepal. Asia Pac J Ophthalmol. 2020;9(1):54–58. doi:10.1097/APO.0000000000000546
31. Kumar S, Gupta S, Sharma A, Wang J-L. Sensitivity and specificity of smartphone-based retinal imaging for diabetic retinopathy: a comparative study. BMC Ophthalmol. 2022;22(1):123. doi:10.1186/s12886-022-02388-1
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.