Back to Journals » Journal of Inflammation Research » Volume 18
The Value of Systemic Inflammatory Index and Nutritional Marker in Predicting Acute Calculus Cholecystitis and Its Severity
Authors He B, He Q, Lai Z, Niu Z, Zhang J, Wang Y
Received 12 March 2025
Accepted for publication 11 July 2025
Published 19 July 2025 Volume 2025:18 Pages 9505—9521
DOI https://doi.org/10.2147/JIR.S521080
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Bing He,1,* Qiguang He,2,* Zhiyong Lai,3 Zhiqiang Niu,3 Jijun Zhang,3 Yingkai Wang3
1Department of First Clinical Medical College, Shanxi Medical University, Taiyuan, Shanxi, People’s Republic of China; 2Department of First Clinical Medical College, China Medical University, Shenyang, Liaoning, People’s Republic of China; 3Department of Biliopancreatic Surgery, The First Hospital of Shanxi Medical University, Taiyuan, Shanxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jijun Zhang; Yingkai Wang, Department of Biliopancreatic Surgery, The First Hospital of Shanxi Medical University, Taiyuan, Shanxi, People’s Republic of China, Email [email protected]; [email protected]
Background: The study aimed to evaluate the accuracy with which various nutritional and inflammatory indicators, including Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), Systemic Immune-Inflammation Index (SII), Monocyte-to-Lymphocyte Ratio (MLR), Systemic Inflammatory Response Index (SIRI), and Prognostic Nutritional Index (PNI), can predict the severity of acute calculus cholecystitis.
Methods: 109 cases of chronic calculus cholecystitis (CCC) and 130 cases of acute calculus cholecystitis (ACC), including 62 cases of acute simple cholecystitis (ASC), 35 cases of acute purulent cholecystitis (APC), and 33 cases of acute gangrenous cholecystitis (AGC), were encompassed in the retrospective cohort study. The patients’ clinical information and inflammatory-immune markers were collected for analysis.
Results: The optimal cut-off values for NLR, SIRI, SII, MLR, PLR, and PNI in distinguishing ACC from CCC were determined to be 2.499, 0.964, 593.5, 0.230, 148.0, and 141.3, respectively. NLR > 2.499 demonstrated the highest predictive capability, with an AUC of 0.896. Multivariate analysis indicated that NLR > 2.499 (OR: 4.69, p = 0.006) was the dominant factor in differentiating ACC from CCC. The optimal cut-off values for SII, SIRI, MLR, NLR, PLR, and PNI in distinguishing ASC from APC were 1098, 2.092, 0.304, 4.082, 191.1, and 135.3, respectively. SII > 1098 exhibited the highest predictive capability, with an AUC of 0.73. The optimal cut-off values for NLR, SIRI, MLR, SII, and PLR in differentiating APC from AGC were 7.232, 4.773, 0.557, 2417, and 221.5, respectively. NLR > 7.232 demonstrated the highest predictive capability, with an AUC of 0.826.
Conclusion: Systemic inflammatory index and nutritional marker can serve as valuable indicators for predicting acute calculus cholecystitis and its severity. An elevated systemic inflammatory index and a declining nutritional marker suggest an increased risk of severe cholecystitis, warranting prompt and appropriate interventions.
Keywords: systemic inflammatory index, nutritional marker, calculus cholecystitis, severity, diagnosis
Introduction
Cholecystitis, a prevalent condition characterized by gallbladder inflammation, occurs in nearly 90% of patients due to cystic duct obstruction, leading to bile stasis and subsequent infection.1–3 The clinical manifestations of calculus cholecystitis vary widely, ranging from chronic calculous cholecystitis (CCC), which can be managed conservatively, to acute calculous cholecystitis (ACC), which may progress to septic shock.4 Clinicians generally recognize the favorable prognosis associated with chronic calculous cholecystitis. However, severe forms of the disease pose a significant threat; delayed treatment can lead to complications such as purulent inflammation, gangrene, and perforation. This highlights the importance of prompt and accurate diagnosis, followed by appropriate therapeutic interventions, to prevent complications and avoid doctor-patient conflicts in inpatients.5
Currently, although the Tokyo Guidelines 2018 (TG18) is a widely accepted standard for assessing the severity of ACC using scoring systems, it primarily relies on a comprehensive medical history, symptoms, physical examination, and imaging findings.6 This approach is time-consuming and may increase the risk of mortality and morbidity due to clinical deterioration and transfer delays. Therefore, rapid and accurate diagnosis and evaluation are crucial for developing the best treatment plan and providing timely and effective care when cholecystitis occurs. A reliable predictive scoring system is essential to enable clinicians to stratify patients based on prognosis and ensure close monitoring of high-risk patients in an intensive care setting.
In recent years, numerous systemic inflammatory indexes, such as NLR (Neutrophil-to-Lymphocyte Ratio), PLR (Platelet-to-Lymphocyte Ratio), SII (Systemic Immune-Inflammation Index), MLR (Monocyte-to-Lymphocyte Ratio), and SIRI (Systemic Inflammatory Response Index), have emerged as potential predictors of the progression of inflammatory diseases and cancer. Research indicates that these systemic inflammatory indexes offer greater sensitivity than the total white blood cell count (WBC) for the diagnosis and stratification of systemic infections, sepsis, and bacteremia.7–9 These indices provide a rapid, accessible, easy-to-calculate, and cost-effective method for differentiating between ACC and CCC and determining the severity of ACC, particularly in developing countries where access to advanced imaging and diagnostic tools may be limited. In this study, we evaluated the utility of systemic inflammatory indexes in predicting acute calculus cholecystitis and its severity.
Inflammation, which is inherently catabolic, is intricately linked to the body’s nutritional status. Nutritional markers play a crucial role in various aspects, including health management, disease prevention, treatment, and clinical nutrition. Notably, indices such as the PNI (Prognostic Nutritional Index), NRI (Nutritional Risk Index), NUTRIC (nutrition risk in the critically ill) score, CONUT (Control of Nutritional Status score) score, and ALB (albumin) score have become essential tools for medical professionals to evaluate patients’ nutritional and immunological health. These markers consider serum albumin levels, lymphocyte counts, and weight fluctuations, facilitating the development of personalized nutritional interventions to enhance patient care and disease management. These indicators are simple to obtain and may reflect the patient’s preoperative physical condition, nutritional status, and inflammation. Predicting gallbladder inflammation based on preoperative nutritional indexes and stratifying the disease can improve the diagnostic efficiency and prognosis of patients. In this study, we also evaluated the value of nutritional markers in predicting acute calculus cholecystitis and its severity.
In our retrospective clinical study, we combined the systemic inflammatory index and the nutritional index to stratify patients with gallstones and cholecystitis to identify the best predictors. Through this study, clinicians can easily and quickly conduct risk assessments of patients with gallstones and cholecystitis before surgery, enabling timely triage and the adoption of personalized treatment plans. Timely treatment of the disease, halting its progression, and improving patient outcomes are of utmost importance.
Clinical Data and Methods
Study Site and Setting
This study was conducted at the First Hospital of Shanxi Medical University. The hospital serves as a major referral center, with patients accessing treatment through two primary routes: local residents seeking care via outpatient services or hospitalization, and out-of-town patients typically referred by other hospitals for specialized care. Patients with calculous cholecystitis are admitted to the hospital after a definitive diagnosis, and a personalized treatment plan is formulated according to the severity of the patient’s condition. Specifically, we clarified the therapeutic protocols according to the Tokyo guidelines as follows: Elective cholecystectomy is recommended for chronic calculous cholecystitis, while early laparoscopic cholecystectomy remains the standard for mild acute cases, including acute simple calculous cholecystitis. For moderate acute presentations (eg, acute purulent or gangrenous calculous cholecystitis), the decision between early or delayed cholecystectomy is determined by an experienced hepatobiliary surgeon based on preoperative judgment and patient stability. In severe acute cholecystitis, initial medical stabilization—including antibiotics and image-guided percutaneous cholecystostomy—is prioritized, followed by delayed surgical intervention.10
Study Population
This study retrospectively analyzed the clinical data of patients with calculous cholecystitis between January 2023 and January 2024. The inclusion criteria were as follows: 1) Age ≥ 18 years; 2) Availability of complete clinical data for analysis. The exclusion criteria included: 1) Patients with concurrent acute inflammatory diseases or malignant tumors; 2) Patients who had received antibiotic treatment and nutritional supportive therapy prior to hospitalization; 3) Patients complicated with gallbladder polyp; 4) Patients complicated with intrahepatic or extrahepatic cholelithiasis; 5) Non-calculous cholecystitis; 6) Patients with missing clinical data.
Data Collection
Patients’ demographic information (Age, Gender, and BMI (kg/m2)), comorbidities (Hypertension and Diabetes), history of smoking and alcohol drinking, and blood test results at admission (platelet count [PLT] (*109/L), white blood cell count [WBC] (*109/L), hemoglobin [Hb] (g/L), neutrophil count [N] (*109/L), lymphocyte count [L] (*109/L), monocyte count [M] (*109/L), alanine aminotransferase [ALT] (U/L), aspartate aminotransferase [AST] (U/L), albumin [Alb] (g/L), total bilirubin [TBIL] (μmol/L), alkaline phosphatase [ALP] (U/L), gamma-glutamyl transpeptidase [GGT] (U/L), serum creatinine [Scr] (μmol/L), prothrombin time [PT] (s), International normalized ratio [INR]) were collected. Systemic Inflammatory Indexes and Nutritional Markers, including NLR, PLR, SII, MLR, SIRI, and PNI, were calculated as follows: NLR (neutrophils/lymphocytes ratio), PLR (platelets/lymphocytes ratio), SII ((neutrophils×platelets)/lymphocytes), MLR (monocytes/lymphocytes ratio), SIRI ((monocytes×neutrophils)/ lymphocytes), PNI (albumin+5×lymphocytes).
Group and Definition
After the gallbladder was removed, histopathological examination was performed by a consultant pathologist. The final diagnosis of calculus cholecystitis was based on the pathological examination of postoperative gallbladder tissue specimens. Calculous cholecystitis includes CCC and ACC, and ACC can be further divided into ASC, APC, and AGC. The details are as follows:
Chronic Calculus Cholecystitis (CCC): Histopathological examination reveals fibrosis and collagenous thickening of the gallbladder wall, accompanied by mucosal atrophy, glandular depletion, and characteristic Rokitansky-Aschoff sinus formation. Chronic inflammatory infiltrates—predominantly lymphocytes and plasma cells—are consistently observed. Advanced cases may exhibit mural calcification (“porcelain gallbladder”). Multiple gallstones, often impacted within the gallbladder lumen, are pathognomonic features of this condition (Figure 1).
Acute Simple Cholecystitis (ASC): Histopathological evaluation demonstrates diffuse edema with mild mural thickening, secondary to vascular congestion and interstitial fluid accumulation. The mucosa exhibits focal ulceration with predominant neutrophilic infiltration, while fibrinous serosal exudation may be observed. A hallmark feature is cystic duct obstruction by impacted calculi, which precipitates the inflammatory cascade. The inflammatory process remains confined to the gallbladder wall, demonstrating an absence of transmural necrosis, abscess formation, or systemic complications. This early-stage presentation is pathognomonically associated with calculi impaction in the cystic duct (Figure 1).
Acute Purulent Cholecystitis (APC): Histopathological evaluation reveals transmural inflammation with microabscess formation within the gallbladder wall and lumen, accompanied by dense neutrophilic infiltrates and necrotic cellular debris. Extensive mucosal ulceration and suppurative exudate are characteristic, while fibrinopurulent adhesions frequently involve the serosal surface. This advanced stage often arises from bacterial proliferation secondary to persistent cystic duct obstruction, representing a progression from acute simple cholecystitis. The condition carries a heightened risk of perforation or systemic infectious complications, necessitating urgent clinical intervention (Figure 1).
Acute Gangrenous Cholecystitis (AGC): Histopathological evaluation demonstrates transmural coagulative necrosis affecting all mural layers (mucosa, muscularis, serosa), accompanied by vascular thrombosis and bacterial colonization. Macroscopic examination reveals green-black discoloration with hemorrhagic foci, indicative of advanced tissue ischemia. Prominent neutrophilic infiltrates and areas of liquefactive necrosis coexist with necrotic debris, while thrombosed vessels underscore the ischemic pathogenesis. This life-threatening condition carries a high risk of gallbladder perforation, potentially precipitating bile peritonitis. Immediate surgical intervention is imperative to mitigate systemic complications (Figure 1).
Statistical Methods
This study performed statistical analyses using R 4.4.2 software. For continuous variables that conformed to the normal distribution, the mean ± standard deviation was used, and the t-test was employed to compare the differences between the two groups. When the data did not satisfy the normal distribution, the median (interquartile range) was used, and the nonparametric (Mann–Whitney) test was applied to compare the differences between the two groups. The chi-square test was used for comparing discontinuous data between groups. The ROC curve was plotted, and the AUC value was calculated using the “pROC” package. The ROC curve analysis was performed to estimate optimal cut-off values, maximizing sensitivity and specificity according to the Youden index. The logistic model was constructed and fitted using the generalized linear model “glm” function, with the fitting method set as the binomial distribution.
Results
Study the Demographic and Clinical Characteristics of Patients
From the initial pool of 285 patients screened for cholecystitis with gallbladder stones with histological evidence, exclusions were made for incomplete clinical data (15 patients), concurrent inflammatory diseases (6 patients), malignant tumors (4 patients), prior antibiotic treatment (9 patients), nutritional supportive therapy (6 patients), and gallbladder polyp complications (6 patients). This resulted in a study cohort of 239 patients (Figure 2). A total of 109 patients with CCC (33 male, 76 female) with a median age of 53.5 years and 130 patients with ACC (59 male, 71 female) with a median age of 58.4 years were included in this study. The values of WBC, N, and M were significantly higher in ACC patients with gallbladder stones compared to CCC patients (P < 0.001). Furthermore, ACC patients had higher TBIL, GGT, and Scr levels compared to CCC patients (P < 0.05). Conversely, ACC patients exhibited decreased concentrations of M and Alb compared to CCC patients (P < 0.001). Notably, patients with ACC had significantly higher levels of systemic inflammatory indexes such as NLR, PLR, SII, MLR, and SIRI but lower levels of nutritional markers such as PNI and Alb compared to CCC patients. Table 1 shows the demographic and baseline clinical characteristics of the study patients.
 |
Table 1 Comparison of Basic Clinical Characteristics Between CCC and ACC |
Comparison of General Data of Patients with Gallbladder Stones with Acute Cholecystitis of Different Severity
ACC patients were retrospectively divided into three groups based on their postoperative pathological diagnosis: ASC, APC, and AGC. The ASC group included 62 cases, consisting of 24 males and 38 females with an average age of 55.5 ± 15.5 years. The APC group included 35 cases, consisting of 14 males and 21 females with an average age of 54.9 ± 16.4 years. The AGC group included 33 cases, consisting of 21 males and 12 females with an average age of 67.4 ± 8.34 years. There were no significant differences in gender, comorbidities, and BMI among patients with varying severity levels (P > 0.05). However, there were statistically significant differences in WBC, N, L, or Alb between the groups (P < 0.05). Additionally, there were apparent differences in systemic inflammatory indexes such as NLR, PLR, SII, MLR, and SIRI (P < 0.05), but no significant differences in the nutritional marker PNI among patients with varying severity levels (P > 0.05). The detailed demographic information and baseline clinical characteristics of patients are shown in Table 2.
 |
Table 2 Comparison of Basic Clinical Characteristics of ASC, APC and AGC Patients |
Using Systemic Inflammatory Index and Nutritional Marker Analyzes Acute Calculus Cholecystitis and Its Severity
Our study further validates the significant value of systemic inflammatory indexes (such as NLR, PLR, SII, MLR, SIRI) in differentiating acute from chronic calculus cholecystitis. The baseline data analysis revealed significant differences in the levels of five inflammatory biomarkers among patients with acute and chronic cholecystitis with cholecystolithiasis. Notably, the values of NLR, SII, and SIRI in the ACC group were significantly higher than those in the CCC group, more than six times as high, indicating that the systemic inflammatory response and immune dysregulation in ACC patients are more pronounced. This may signal the severity of the disease and a poor clinical prognosis. The data variability among patients with acute calculus cholangitis was considerable, suggesting significant inter-group differences in immune responses and inflammatory control, which may reflect the instability of the body under different infection conditions. Table 2 corroborated these findings, showing that the preoperative NLR, PLR, SII, MLR, and SIRI increased as the severity of acute calculus cholecystitis worsened (P < 0.001). Additionally, the preoperative PT and INR lengthened as the severity of acute calculus cholecystitis worsened, which may reflect the blood clotting disorder of the body under different infection conditions. Conversely, nutritional indicators such as PNI and Alb trended downward as the severity escalated, which may reflect the poor nutritional status of the body as the severity of acute calculus cholecystitis increased.
Evaluation of the Differential Ability of Systemic Inflammatory Index and Nutritional Marker in Differentiating Acute from Chronic Calculus Cholecystitis
Addressing the challenges of accurately classifying acute from chronic calculus cholecystitis and the lack of standardized laboratory references, our study employed ROC analysis for both systemic inflammatory and nutritional indexes. The ROC curves, illustrated in Figure 3A, revealed that the AUCs for NLR, SIRI, SII, PLR, MLR, Alb, and PNI were different, and the AUC of NLR was the largest. The optimal cut-off value of NLR in distinguishing acute from chronic calculus cholecystitis was 2.499. The sensitivity at this cut-off point was 74.6%, the specificity was 94.4%, and the AUC was 0.896. Notably, NLR emerged as the superior inflammatory index, consistent with other research and affirming the validity of our optimal threshold.11 The optimal cut-off value of SIRI in distinguishing acute from chronic calculus cholecystitis was 0.964. The sensitivity at this cut-off point was 72.3%, the specificity was 90.8%, and the AUC was 0.875. The optimal cut-off value of SII in distinguishing acute from chronic calculus cholecystitis was 593.5. The sensitivity at this cut-off point was 71.5%, the specificity was 93.5%, and the AUC was 0.864. The optimal cut-off value of MLR in distinguishing acute from chronic calculus cholecystitis was 0.230. The sensitivity at this cut-off point was 73.8%, the specificity was 88.1%, and the AUC was 0.855. The optimal cut-off value of PLR in distinguishing acute from chronic calculus cholecystitis was 148.0. The sensitivity at this cut-off point was 61.5%, the specificity was 83.4%, and the AUC was 0.755. The optimal cut-off value of Alb in distinguishing acute from chronic calculus cholecystitis was 39.45. The sensitivity at this cut-off point was 46.9%, the specificity was 82.5%, and the AUC was 0.629. The optimal cut-off value of PNI in distinguishing acute from chronic calculus cholecystitis was 141.3. The sensitivity at this cut-off point was 48.4%, the specificity was 66.9%, and the AUC was 0.581. This may be related to the fact that in the early stages of acute inflammation, the albumin in the body has not been chronically depleted, and although the lymphocyte count has changed, the PNI has not changed significantly, resulting in a relatively low AUC efficacy.12 These factors, despite their varying accuracy, can aid in identifying acute from chronic cholecystitis with gallbladder stones at specific cut-offs, as shown in Table 3.
 |
Table 3 ROC Curve Analysis for Systemic Inflammatory Index and Nutritional Marker Between CCC and ACC Patients |
Conducting Logistic Regression Analyses to Evaluate the Clinical Significance of Systemic Inflammatory Index and Nutritional Marker as Biomarkers in Distinguishing Between ACC and CCC Patients
Univariate and multivariate logistic analyses were conducted to evaluate the clinical significance of systemic inflammatory index and nutritional marker as biomarkers in distinguishing between ACC and CCC patients. Variables that demonstrated a P - value of < 0.05 in the univariate analysis were subsequently included in the multivariable binary logistic analysis, encompassing age, gender, WBC, Alb, TBIL, GGT, Scr, PT, NLR, PLR, SII, PNI, and SIRI. Notably, in the multivariate analysis, only one variable was found to be significant in discriminating between ACC and CCC, which is NLR > 2.499 (OR: 4.69, P = 0.006). As shown in Table 4.
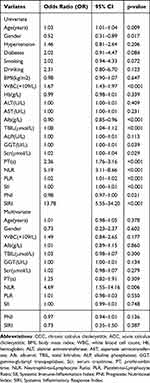 |
Table 4 Univariate and Multivariate Logistic Regression Analysis for the Factors Distinguishing ACC From CCC Patients |
Evaluation of the Differential Ability of Systemic Inflammatory Index and Nutritional Marker as Biomarkers in Predicting the Severity of Acute Cholecystitis with Cholecystolithiasis (Acute Simple Cholecystitis VS Acute Purulent Cholecystitis)
Addressing the challenges of accurately classifying acute simple cholecystitis from acute purulent cholecystitis with cholecystolithiasis, our study employed ROC analysis for both inflammatory and nutritional indexes. The ROC curves, illustrated in Figure 3B, revealed that the AUCs for SII, SIRI, MLR, NLR, PLR, Alb, and PNI were different, and the AUC of SII was the largest. The optimal cut - off value of SII in distinguishing acute simple cholecystitis from acute purulent cholecystitis with cholecystolithiasis was 1098. The sensitivity at this cut - off point was 60%, the specificity was 82.2%, and the AUC was 0.73. Notably, SII emerged as the superior inflammatory index, which may be related to the transformation from local inflammation in ASC to systemic inflammation in APC.13 The optimal cut - off value of SIRI in distinguishing acute simple cholecystitis from acute purulent cholecystitis with cholecystolithiasis was 2.092. The sensitivity at this cut - off point was 65.7%, the specificity was 80.6%, and the AUC was 0.728. The optimal cut - off value of MLR in distinguishing acute simple cholecystitis from acute purulent cholecystitis with cholecystolithiasis was 0.304. The sensitivity at this cut - off point was 65.7%, the specificity was 69.3%, and the AUC was 0.697. The optimal cut - off value of NLR in distinguishing acute simple cholecystitis from acute purulent cholecystitis with cholecystolithiasis was 4.082. The sensitivity at this cut - off point was 62.8%, the specificity was 79%, and the AUC was 0.696. The optimal cut - off value of PLR in distinguishing acute simple cholecystitis from acute purulent cholecystitis with cholecystolithiasis was 191.1. The sensitivity at this cut - off point was 51.4%, the specificity was 79%, and the AUC was 0.636. The optimal cut - off value of Alb in distinguishing acute simple cholecystitis from acute purulent cholecystitis with cholecystolithiasis was 39.85. The sensitivity at this cut - off point was 54.2%, the specificity was 66.1%, and the AUC was 0.556. The optimal cut - off value of PNI in distinguishing acute simple cholecystitis from acute purulent cholecystitis with cholecystolithiasis was 135.3. The sensitivity at this cut - off point was 42.8%, the specificity was 70.9%, and the AUC was 0.54. These factors, despite their varying accuracy, can aid in identifying acute simple cholecystitis from acute purulent cholecystitis with gallbladder stones at specific cut - offs, as shown in Table 5.
 |
Table 5 ROC Curve Analysis for Systemic Inflammatory Index and Nutritional Marker Between ASC and APC Patients |
Evaluation of the Differential Ability of Systemic Inflammatory Index and Nutritional Marker as Biomarkers in Predicting the Severity of Acute Cholecystitis with Cholecystolithiasis (Acute Purulent Cholecystitis vs Acute Gangrenous Cholecystitis)
Addressing the challenges of accurately classifying acute purulent cholecystitis from acute gangrenous cholecystitis with cholecystolithiasis, our study employed ROC analysis for both inflammatory and nutritional indexes. The ROC curves, illustrated in Figure 3C, revealed that the AUCs for NLR, SIRI, MLR, SII, Alb, PLR, and PNI were different, and the AUC of NLR was the largest. The optimal cut - off value of NLR in distinguishing acute purulent cholecystitis from acute gangrenous cholecystitis with cholecystolithiasis was 7.232. The sensitivity at this cut - off point was 84.8%, the specificity was 68.5%, and the AUC was 0.826. The optimal cut - off value of SIRI in distinguishing acute purulent cholecystitis from acute gangrenous cholecystitis with cholecystolithiasis was 4.773. The sensitivity at this cut - off point was 78.7%, the specificity was 74.2%, and the AUC was 0.801. The optimal cut - off value of MLR in distinguishing acute purulent cholecystitis from acute gangrenous cholecystitis with cholecystolithiasis was 0.557. The sensitivity at this cut - off point was 72.7%, the specificity was 77.1%, and the AUC was 0.799. The optimal cut - off value of SII in distinguishing acute purulent cholecystitis from acute gangrenous cholecystitis with cholecystolithiasis was 2417. The sensitivity at this cut - off point was 63.6%, the specificity was 85.7%, and the AUC was 0.759. The optimal cut - off value of Alb in distinguishing acute purulent cholecystitis from acute gangrenous cholecystitis with cholecystolithiasis was 37.15. The sensitivity at this cut - off point was 69.6%, the specificity was 77.1%, and the AUC was 0.718. The optimal cut - off value of PLR in distinguishing acute purulent cholecystitis from acute gangrenous cholecystitis with cholecystolithiasis was 221.5. The sensitivity at this cut - off point was 63.6%, the specificity was 71.4%, and the AUC was 0.683. However, the AUC of PNI was relatively small and was only 0.462, which may be associated with a high proportion of neutrophils in the total number of leukocytes and fewer lymphocytes in severe inflammation, resulting in no significant difference between APC and AGC. As shown in Table 6.
 |
Table 6 ROC Curve Analysis for Systemic Inflammatory Index and Nutritional Marker Between APC and AGC |
Discussion
Cholecystitis typically manifests symptoms such as a positive Murphy’s sign, pain, and tenderness in the right upper quadrant.14 In fact, the etiology of cholecystitis is multifactorial and interrelated. Research indicates that approximately 95% of cholecystitis cases are associated with gallstones.15 Calculus cholecystitis, characterized by inflammation of the gallbladder, is typically triggered by blockage of the cystic duct. It often has a protracted course and a high risk of recurrence.1,16,17 Delayed intervention can lead to chronic cystic bile duct obstruction, resulting in gallbladder lumen dilation and wall thickening due to submucosal edema. This can exacerbate the physical and psychological burden on patients and complicate treatment. Therefore, timely intervention is crucial for preventing further complications.18,19
However, cholecystitis with gallstones cannot be definitively diagnosed by a single examination currently. Diagnosis mainly relies on the traditional TG18 diagnostic criteria, which encompass a series of complex standards and indicators. A comprehensive evaluation of the patient’s clinical manifestations, laboratory findings, and radiological results is required.6,20 Although TG18 is regarded as a benchmark for assessing cholecystitis with gallstones, it demands a comprehensive assessment of the entire body and consumes a significant amount of time to implement. Consequently, these guidelines may not be expedient for accurately and rapidly diagnosing different types of cholecystitis in the preoperative period in emergent clinical scenarios.
Therefore, we analyzed the role of systemic inflammatory indexes and nutritional markers in predicting acute calculus cholecystitis and its severity, aiming to enable clinicians to quickly determine the severity of a patient’s condition and make optimal decisions.
A plethora of studies have introduced diverse predictive models for different types of cholecystitis, incorporating risk factors such as thiol - disulfide, ischemia - modified albumin, monocyte distribution width (MDW), neutrophil - to - lymphocyte ratio (NLR), C - reactive protein (CRP), platelet - to - lymphocyte ratio (PLR), and systemic immune - inflammation index (SII). Melih et al conducted an extensive analysis involving 108 patients hospitalized with acute cholecystitis and 42 healthy volunteers, highlighting the role of oxidative stress markers in assisting radiologic examinations to determine the severity of acute cholecystitis.21 In another study, Kao et al examined data from 331 patients and deduced that patients with an MDW≥21.6 had a higher risk of severe cholecystitis and were more likely to have a prolonged hospital stay.22
Additionally, a retrospective cohort study including 160 elderly patients with cholecystolithiasis and cholecystitis (45 cases of simple cholecystitis, 58 cases of suppurative cholecystitis, 57 cases of gangrenous cholecystitis) and 60 cases of normal gallbladder histology indicated that preoperative NLR and CRP levels could be used to distinguish cholecystolithiasis with cholecystitis in elderly patients. Preoperative NLR levels could also be used to distinguish simple cholecystitis from purulent cholecystitis, as well as purulent cholecystitis from gangrenous cholecystitis in elderly patients with cholecystolithiasis.23 Serban et al, by analyzing the value of NLR, PLR, and SII in predicting advanced inflammation, the risk of conversion, and postoperative complications of acute calculous cholecystitis, concluded that NLR was superior to PLR and SII.13
However, the quantification of oxidative stress markers, monocyte distribution width, and CRP is frequently hindered by the limitations of hospital equipment, preventing many healthcare facilities from conducting such evaluations. This limitation underscores the urgent need for an economically feasible and universally applicable standard that can be seamlessly integrated into routine clinical practice to precisely assess the severity of cholecystitis with gallstones. Li et al utilized combined scoring systems (neutrophil - to - lymphocyte ratio and prognostic nutritional index) to simply and accurately predict the severity of inpatient acute cholangitis, thereby improving the accuracy and efficiency of clinical decision - making in complex cases and for high - risk patients.24
Confronted with the aforementioned diagnostic challenges, our team combined systemic inflammatory indexes with nutritional markers to analyze and predict acute calculus cholecystitis and its severity. As the disease severity escalates, inflammatory biomarkers such as NLR, PLR, SII, MLR, and SIRI increase significantly, whereas nutritional indicators like PNI and Alb decline markedly.
Our research revealed that various systemic inflammatory indexes and nutritional markers derived from a complete blood count are prognostic tools for ascertaining the severity of cholecystitis with cholecystolithiasis. They enable clinicians to rapidly differentiate the severity levels of cholecystitis with cholecystolithiasis using basic test data, thus facilitating the interpretation of risk assessments for both physicians and patients. This straightforward and expedient method enhances doctor - patient communication, ensures the prompt recognition of cholecystitis with cholecystolithiasis, and informs tailored medical decisions for patients with diverse clinical presentations. Notably, for patients with acute purulent cholecystitis and acute gangrenous cholecystitis, the expedited transfer to ICU wards has been instrumental in saving critical time, thereby reducing the mortality rates associated with the condition.
Previous studies have elucidated that white blood cell count and leukocyte subtype cells are valuable indicators for predicting the inflammatory process in acute cholecystitis.25,26 The white blood cell count has been established as the primary biomarker for assessing the severity of cholecystitis.6 In recent years, with the continuous improvement of the requirements for the accuracy of disease diagnosis, a large number of systemic inflammatory indexes have emerged, including NLR, PLR, SII, MLR, and SIRI. These systemic inflammatory indexes, calculated using neutrophils, lymphocytes, platelets, and monocytes, greatly improve the diagnostic accuracy and efficiency of inflammatory diseases such as acute cholecystitis, acute cholangitis, acute pancreatitis, and pneumonia.24,26–28
Neutrophils, which are pivotal in orchestrating inflammatory responses, exacerbate systemic inflammation through the secretion of pro - inflammatory cytokines. In severe acute cholangitis cases, this can potentially precipitate Systemic Inflammatory Response Syndrome (SIRS) and septic shock.29 Concurrently, lymphocytes modulate this systemic inflammation. Persistent inflammatory states induce lymphocyte redistribution and apoptosis, thereby decreasing lymphocyte counts.30 Numerous studies have shown that elevated platelet counts are associated with poor prognosis in many inflammatory diseases and cancers, including lung, gastric, pancreatic, cervical, ovarian, and prostate inflammations and cancers.31–35 Monocytes are a key component of both the innate and adaptive immune systems. They circulate in the peripheral blood and migrate into tissues, where they can differentiate into tissue macrophages.36,37 Monocytes and their macrophage derivatives play an important role in the regulation of immune - inflammatory responses.38,39
In our study, we identified systemic inflammatory indexes as potential predictors for the preoperative diagnosis and postoperative pathological classification of cholecystitis. Previous research also supports our findings. In a retrospective study, Chen et al evaluated 105 chronic calculous cholecystitis (CCC) and 88 acute calculous cholecystitis (ACC) patients and concluded that systemic inflammation indexes, including NLR, PLR, and SII, can be useful in predicting the risk of mild - degree ACC and moderate - to - severe - degree ACC. NLR can serve as an optimum indicator with distinguishing power and sensitivity, differentiating ACC from CCC.11
Consistent with our findings, a study by Lee et al observed a significant association between NLR and acute gangrenous cholecystitis (AGC), where each standard deviation increase in NLR corresponded to a 48% heightened probability of AGC.40 In another retrospective study, Turhan et al indicated that the PLR level was significantly higher in the complicated cholecystitis group, and the best cutoff value for PLR was 146.9, which is consistent with our research.41 In a prospective study, NLR>5 and PLR>175 can serve as inflammatory biomarkers, assisting physicians in the clinical diagnosis of acute gangrenous cholecystitis.42
In previous studies, Cakcak et al, retrospectively recruiting 126 consecutive patients with acute cholecystitis with and without cholecystostomy, found that systemic inflammatory indexes, including NLR, PLR, SII, and SIRI, are significantly elevated in patients with cholecystostomy. They deduced that systemic inflammatory indexes at the time of admission could help in the predictive evaluation of the treatment of patients with acute cholecystitis.43 Additionally, Dincer et al found that calculating the MLR at the time of admission could help in the predictive evaluation of the treatment of patients with acute cholecystitis.44
An increasing number of current studies support the link between nutritional markers and the severity of the disease and its prognosis. The Nutritional Risk Index (NRI) is derived from weight change and blood albumin levels and is lauded for its ability to assess the malnutrition risk in various patient populations. However, for specific patient subgroups, it may not be sensitive enough or fully applicable. The NUTRIC score, designed specifically for patients in the intensive care unit (ICU), includes easily obtainable and objective characteristics.45 However, its correlation with accurate indicators of nutritional status remains weak.
The CONUT score (Control of Nutritional Status score) enables the early detection of malnutrition. It measures nutritional status by assessing blood albumin, cholesterol, and lymphocyte count.46 However, there are several limitations to its wider application across different disorders. The serum albumin score, or ALB score, a simple blood test, reflects a patient’s liver function and nutritional state. However, confounding variables such as edema or hepatic disease can affect its predictive accuracy.47
The Prognostic Nutritional Index (PNI) reflects patients’ nutritional state and is well - known for its predictive accuracy. It functions as a dual predictor of immune function and nutrition. PNI is frequently used to predict mortality and surgical complications; worse scores are associated with unfavorable outcomes. Systemic inflammatory responses, characterized by increased vascular permeability, facilitate albumin transfer from the intravascular compartment. Inflammation suppresses hepatic albumin mRNA expression, slowing albumin synthesis and accelerating its breakdown. While inflammatory cytokines, particularly IL - 6, promote the production of acute - phase proteins like C - reactive protein, they also inhibit hepatocyte albumin synthesis.48
The NF - κB pathway is a key component of the inflammatory signaling pathways that regulate lymphocyte dynamics. When pathogens or tumor necrosis factors activate it, pro - inflammatory cytokines like IL - 1 and IL - 6 are upregulated, which may lead to lymphocytopenia.49 Furthermore, during the stress response induced by inflammation, immune - suppressive chemicals such as corticosteroids are released, which further exacerbates the immune - suppressive state by inhibiting lymphocyte formation.50
A recent prospective study found an inverse linear relationship between pre - diagnostic serum albumin levels and the risk of inflammatory diseases, particularly in patients with acute cholangitis, multiple myeloma, etc.24,51 This was further supported by a retrospective study by Li et al, which noted that PNI might be a significant predictive factor for acute cholangitis.24 Empirical investigations have shown that both PNI and the Control of Nutritional Status (CONUT) score influence the survival of multiple myeloma patients; a low PNI is predictive of a poor prognosis.51 Another retrospective investigation that examined patients who had surgery for secondary spontaneous pneumothorax showed that the predictive nutritional index was significantly positively and negatively correlated with body mass index and performance status, respectively. This study also demonstrated that a lower prognostic nutritional index was an independent risk factor for postoperative complications.52
However, the relationship between PNI changes and the severity of calculus cholecystitis was previously unknown. Our study explored this association by using PNI as a predictive tool for calculus cholecystitis. In our study, the PNI was relatively low in predicting acute calculous cholecystitis and its severity, probably because the protein in the body was not overly depleted during acute inflammation, resulting in relatively little difference between groups. In the future, the relationship between PNI and calculous cholecystitis still needs to be further determined by large - sample randomized controlled trials.
In the field of intensive care, procalcitonin (PCT) and C - reactive protein (CRP) have emerged as prominent biomarkers. In a single - center retrospective study involving 401 patients, the results showed that CRP and PCT are independent risk factors for suppurative bile and protract nomogram, with an area under the curve of 0.875, a sensitivity of 86.6%, and a specificity of 75.5%.53 Despite its predictive ability, routine testing for serum procalcitonin is not common in all medical institutions, mainly due to its high cost, which limits its continuous use during hospitalization.54 CRP, initially used for assessing disease severity and monitoring conditions such as rheumatoid arthritis and secondary amyloidosis, has seen its role in clinical practice decline in favor of more cost - effective and easily applied markers such as CRP and erythrocyte sedimentation rate (ESR).55 At The First Hospital of Shanxi Medical University, PCT and CRP have not been established as the primary tests for patients admitted with calculus cholangitis, leaving their potential for predicting the severity of the condition an open question for future research.
However, our study has several limitations. First, it is a single - center retrospective study with a relatively small sample size. Second, the patients were exclusively from our institution, and external validation was not performed. Third, we had strict exclusion criteria, limiting the generalizability of our findings to broader populations. Therefore, future research should be conducted as large - scale, multicenter prospective studies with external validation to enhance the reliability and scientific robustness of the findings. Despite these limitations, our study confirms the role of systemic inflammatory indexes and nutritional markers in predicting acute calculus cholecystitis and its severity, facilitating the development of personalized treatment and follow - up strategies.
Conclusion
Our investigation reveals that upon hospital admission, systemic inflammatory index and nutritional marker can be rapidly and cost-effectively determined. These indicators enhance the predictive accuracy for acute calculus cholecystitis and its severity. An elevated systemic inflammatory index and a declining nutritional marker indicate an increased risk of severe cholecystitis, necessitating prompt and appropriate interventions. Such interventions may include conservative management with antibiotics, PTGD (percutaneous transhepatic gallbladder drainage), early or delayed surgical procedures, or expedited transfer to an ICU.
Ethics Approval and Consent to Participate
The study was in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of The First Hospital of Shanxi Medical University (approval number: IIT-2025-031). After obtaining ethical approval, we retrospectively collected the clinical data of patients with calculous cholecystitis between January 2023 and January 2024. Due to the study’s retrospective design, the requirement for informed consent was waived, as confirmed by the Ethics Committee of The First Hospital of Shanxi Medical University. As an analysis of pre-existing medical records, the research posed no more than minimal risk to participants, involved no interventions, and utilized fully anonymized and de-identified data. Researchers additionally signed a clinical research confidentiality commitment to safeguard patient data confidentiality. These measures ensured patient rights and confidentiality were fully protected throughout the investigation.
Acknowledgments
No funding was received for this study. The authors thank biliopancreatic surgery of The First Hospital of Shanxi Medical University for the valuable support of this work.
Author Contributions
All authors made a significant contribution to the work reported, whether it was in the conception, study design, execution, data acquisition, analysis, and interpretation, or all these areas. All authors took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wadhwa V, Jobanputra Y, Garg SK, Patwardhan S, Mehta D, Sanaka MR. Nationwide trends of hospital admissions for acute cholecystitis in the United States. Gastroenterol Rep. 2017;5(1):36–42. doi:10.1093/gastro/gow015
2. Kurihara H, Binda C, Cimino MM, Manta R, Manfredi G, Anderloni A. Acute cholecystitis: which flow-chart for the most appropriate management? Dig Liver Dis. 2023;55(9):1169–1177. doi:10.1016/j.dld.2023.02.005
3. Costanzo ML, D’Andrea V, Lauro A, Bellini MI. Acute cholecystitis from biliary lithiasis: diagnosis, management and treatment. Antibiotics. 2023;12(3). doi:10.3390/antibiotics12030482
4. Global Index Medicus. Guidelines for diagnosis and treatment of acute biliary tract infections(2021). Zhonghua Wai Ke Za Zhi. 2021;59(6):422–429. doi:10.3760/cma.j.cn112139-20210421-00180
5. Mukai S, Itoi T. Requirement of drainage strategy for acute cholecystitis in patients with high surgical risk. Dig Endosc. 2023;35(5):668–669. doi:10.1111/den.14536
6. Yokoe M, Hata J, Takada T, et al. Tokyo guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25(1):41–54. doi:10.1002/jhbp.515
7. Woo SH, Lee WJ, Seol SH, Kim DH, Choi SP. The accuracies of abdominal computed tomography and the neutrophil-to-lymphocyte ratio used to predict the development of clinically severe acute cholecystitis in elderly patients visiting an emergency department. Niger J Clin Pract. 2018;21(5):645–652. doi:10.4103/njcp.njcp_76_17
8. Jeon TJ, Park JY. Clinical significance of the neutrophil-lymphocyte ratio as an early predictive marker for adverse outcomes in patients with acute pancreatitis. World J Gastroenterol. 2017;23(21):3883–3889. doi:10.3748/wjg.v23.i21.3883
9. Kong W, He Y, Bao H, Zhang W, Wang X. Diagnostic value of neutrophil-lymphocyte ratio for predicting the severity of acute pancreatitis: a meta-analysis. Dis Markers. 2020;2020:9731854. doi:10.1155/2020/9731854
10. Lee SW, Yang SS, Chang CS, Yeh HJ. Impact of the Tokyo guidelines on the management of patients with acute calculous cholecystitis. J Gastroenterol Hepatol. 2009;24(12):1857–1861. doi:10.1111/j.1440-1746.2009.05923.x
11. Chen L, Chen X. The role of different systemic inflammatory indexes derived from complete blood count in differentiating acute from chronic calculus cholecystitis and predicting its severity. J Inflamm Res. 2024;17:2051–2062. doi:10.2147/JIR.S453146
12. Akça H, Özkan A, Özdemir S, Özdemir S. The ability of the prognostic nutritional index to predict short-term mortality in geriatric acute heart failure. Egypt Heart J. 2025;77(1):3. doi:10.1186/s43044-024-00604-0
13. Serban D, Stoica PL, Dascalu AM, et al. The significance of preoperative Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Systemic Inflammatory Index (SII) in predicting severity and adverse outcomes in acute calculous cholecystitis. J Clin Med. 2023;12(21):6946. doi:10.3390/jcm12216946
14. Wu XB, Zhong JL, Wang SW, et al. Neutrophil-to-lymphocyte ratio is associated with circumferential wall enhancement of unruptured intracranial aneurysm. Front Neurol. 2022;13:879882. doi:10.3389/fneur.2022.879882
15. Tran A, Hoff C, Polireddy K, Neymotin A, Maddu K. Beyond acute cholecystitis-gallstone-related complications and what the emergency radiologist should know. Emerg Radiol. 2022;29(1):173–186. doi:10.1007/s10140-021-01999-y
16. Chhoda A, Mukewar SS, Mahadev S. Managing gallstone disease in the elderly. Clin Geriatr Med. 2021;37(1):43–69. doi:10.1016/j.cger.2020.08.005
17. Maddu K, Phadke S, Hoff C. Complications of cholecystitis: a comprehensive contemporary imaging review. Emerg Radiol. 2021;28(5):1011–1027. doi:10.1007/s10140-021-01944-z
18. Adachi T, Eguchi S, Muto Y. Pathophysiology and pathology of acute cholecystitis: a secondary publication of the Japanese version from 1992. J Hepatobiliary Pancreat Sci. 2022;29(2):212–216. doi:10.1002/jhbp.912
19. Choudhury SR, Gupta P, Garg S, Kalra N, Kang M, Sandhu MS. Image-guided percutaneous cholecystostomy: a comprehensive review. Ir J Med Sci. 2022;191(2):727–738. doi:10.1007/s11845-021-02655-7
20. Kiriyama S, Kozaka K, Takada T, et al. Tokyo guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25(1):17–30. doi:10.1002/jhbp.512
21. Gül MC, Çetin R, Zihni İ, et al. A marker for acute cholecystitis severity: thiol-disulfide balance and ischemia-modified albumin. Cir Cir. 2024;93(1):59–67. doi:10.24875/CIRU.24000150
22. Kao CH, Liu YH, Chen WK, et al. Value of monocyte distribution width for predicting severe cholecystitis: a retrospective cohort study. Clin Chem Lab Med. 2023;61(10):1850–1857. doi:10.1515/cclm-2023-0195
23. Xia Z, Liu Y, Sun S, Shan E, Liu Y. The value of preoperative neutrophil/lymphocyte ratio in predicting the severity of cholecystolithiasis with cholecystitis in elderly patients. BMC Surg. 2023;23(1):360. doi:10.1186/s12893-023-02267-1
24. Li D, Sun J, Qi C, Fu X, Gao F. Predicting severity of inpatient acute cholangitis: combined neutrophil-to-lymphocyte ratio and prognostic nutritional index. BMC Gastroenterol. 2024;24(1):468. doi:10.1186/s12876-024-03560-w
25. Micić D, Stanković S, Lalić N, Đukić V, Polovina S. Prognostic value of preoperative neutrophil-to-lymphocyte ratio for prediction of severe cholecystitis. J Med Biochem. 2018;37(2):121–127. doi:10.1515/jomb-2017-0063
26. Chen J, Gao Q, Huang X, Wang Y. Prognostic clinical indexes for prediction of acute gangrenous cholecystitis and acute purulent cholecystitis. BMC Gastroenterol. 2022;22(1):491. doi:10.1186/s12876-022-02582-6
27. Li X, Zhang Y, Wang W, et al. An inflammation-based model for identifying severe acute pancreatitis: a single-center retrospective study. BMC Gastroenterol. 2024;24(1):63. doi:10.1186/s12876-024-03148-4
28. Zeba S, Surbatovic M, Udovicic I, et al. Immune cell-based versus albumin-based ratios as outcome predictors in critically Ill COVID-19 patients. J Inflamm Res. 2025;18:73–90. doi:10.2147/JIR.S488972
29. Tahtaci M, Koseoglu H, Alisik M, et al. Association of low fecal elastase-1 and non-ulcer dyspepsia. J Clin Med. 2018;7(6):155. doi:10.3390/jcm7060155
30. Hotchkiss RS, Nicholson DW. Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol. 2006;6(11):813–822. doi:10.1038/nri1943
31. Cho MS, Bottsford-Miller J, Vasquez HG, et al. Platelets increase the proliferation of ovarian cancer cells. Blood. 2012;120(24):4869–4872. doi:10.1182/blood-2012-06-438598
32. Erpenbeck L, Schön MP. Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood. 2010;115(17):3427–3436. doi:10.1182/blood-2009-10-247296
33. Pedersen LM, Milman N. Prognostic significance of thrombocytosis in patients with primary lung cancer. Eur Respir J. 1996;9(9):1826–1830. doi:10.1183/09031936.96.09091826
34. Scholz HS, Petru E, Gücer F, Haas J, Tamussino K, Winter R. Preoperative thrombocytosis is an independent prognostic factor in stage III and IV endometrial cancer. Anticancer Res. 2000;20(5c):3983–3985.
35. Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366(7):610–618. doi:10.1056/NEJMoa1110352
36. Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27(1):669–692. doi:10.1146/annurev.immunol.021908.132557
37. Grage-Griebenow E, Flad HD, Ernst M. Heterogeneity of human peripheral blood monocyte subsets. J Leukoc Biol. 2001;69(1):11–20. doi:10.1189/jlb.69.1.11
38. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–896. doi:10.1038/ni.1937
39. Takaba J, Mishima Y, Hatake K, Kasahara T. Role of bone marrow-derived monocytes/macrophages in the repair of mucosal damage caused by irradiation and/or anticancer drugs in colitis model. Mediators Inflamm. 2010;2010:634145. doi:10.1155/2010/634145
40. Lee SK, Lee SC, Park JW, Kim SJ. The utility of the preoperative neutrophil-to-lymphocyte ratio in predicting severe cholecystitis: a retrospective cohort study. BMC Surg. 2014;14(1):100. doi:10.1186/1471-2482-14-100
41. Turhan VB, Gök HF, Ünsal A, Akpınar M, Güler Şimşek G, Buluş H. Pre-operative neutrophil/lymphocyte and platelet/lymphocyte ratios are effective in predicting complicated acute cholecystitis. Ulus Travma Acil Cerrahi Derg. 2022;28(4):471–476. doi:10.14744/tjtes.2021.49956
42. Díez Ares J, Martínez García R, Estellés Vidagany N, et al. Can inflammatory biomarkers help in the diagnosis and prognosis of gangrenous acute cholecystitis? A prospective study. Rev Esp Enferm Dig. 2021;113(1):41–44. doi:10.17235/reed.2020.7282/2020
43. Cakcak İE, Kula O. Predictive evaluation of SIRI, SII, PNI, and GPS in cholecystostomy application in patients with acute cholecystitis. Ulus Travma Acil Cerrahi Derg. 2022;28(7):940–946. doi:10.14744/tjtes.2022.90249
44. Dincer HA, Cennet O, Dogrul AB. The utility of systemic immune inflammatory index in discriminating between gallbladder cancer and xanthogranulomatous cholecystitis: a single-tertiary center experience. Medicine. 2023;102(43):e35805. doi:10.1097/MD.0000000000035805
45. Yildirim M, Yildirim ZS, Deniz M. Effect of the modified NUTRIC score in predicting the prognosis of patients admitted to intensive care units. BMC Anesthesiol. 2024;24(1):473. doi:10.1186/s12871-024-02866-2
46. Popp D, Stich-Regner M, Schmoelz L, Silvaieh S, Heisinger S, Nia A. Predictive feasibility of the Graz malnutrition screening, controlling nutritional status score, geriatric nutritional risk Index, and prognostic nutritional index for postoperative long-term mortality after surgically treated proximal femur fracture. Nutrients. 2024;16(24):4280. doi:10.3390/nu16244280
47. Bucurica S, Parolă I, Vasile AG, Maniu I, Mititelu MR. The impact of hepatic hydrothorax on the outcome of liver cirrhosis: a comparative study. J Clin Med. 2025;14(1):212. doi:10.3390/jcm14010212
48. Hu D, Huang Z, Li W, et al. Macrophage membrane-cloaked ROS-responsive albumin nanoplatforms for targeted delivery of curcumin to alleviate acute liver injury. Mol Pharm. 2025;22(2):771–786. doi:10.1021/acs.molpharmaceut.4c00808
49. Abbasifard M, Khorramdelazad H. The bio-mission of interleukin-6 in the pathogenesis of COVID-19: a brief look at potential therapeutic tactics. Life Sci. 2020;257:118097.
50. Chen D, Sun F, Miao M, et al. Dose effect of corticosteroids on peripheral lymphocyte profiles in patients with systemic lupus erythematosus. Clin Rheumatol. 2025;44(2):669–679.
51. Ferraro MP, Gimeno-Vazquez E, Subirana I, et al. Anthracycline-induced cardiotoxicity in diffuse large B-cell lymphoma: NT-proBNP and cardiovascular score for risk stratification. Eur J Haematol. 2019;102(6):509–515. doi:10.1111/ejh.13234
52. Shiotani T, Kondo K, Furukawa S, Watanabe M. Usefulness of preoperative prognostic nutritional index in secondary spontaneous pneumothorax. Ann Thorac Surg Short Rep. 2024;2(4):624–628. doi:10.1016/j.atssr.2024.06.016
53. He YQ, Wang H, Zhao YH, et al. Nomogram for assistant diagnosing acute suppurative cholangitis: a case-control study. BMC Gastroenterol. 2024;24(1):322. doi:10.1186/s12876-024-03379-5
54. Shim J, Muraru S, Dobrota R, Fleisch E, Distler O, Barata F. Noninvasive, multimodal inflammatory biomarker discovery for systemic inflammation (NOVA Study): protocol for a cross-sectional study. JMIR Res Protoc. 2024;13:e62877. doi:10.2196/62877
55. Mouliou DS. C-reactive protein: pathophysiology, diagnosis, false test results and a novel diagnostic algorithm for clinicians. Diseases. 2023;11(4).
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




