Back to Journals » Journal of Pain Research » Volume 18
Transforming Chronic Pain Management: Integrating Neuromodulation with Advanced Technologies to Tackle Cognitive Dysfunction – A Narrative Review
Authors Green M, Hayley A , Gunnersen JM , Nazemian V , Cabble A, Thompson S, Chakravarthy K
Received 21 January 2025
Accepted for publication 13 May 2025
Published 16 May 2025 Volume 2025:18 Pages 2497—2507
DOI https://doi.org/10.2147/JPR.S514285
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Dawood Sayed
Maja Green,1,2 Amie Hayley,3 Jenny M Gunnersen,4 Vida Nazemian,1,2 Adam Cabble,1,2 Sandra Thompson,1 Krishnan Chakravarthy1,2
1Clinical Research Organization, Solaris Research Institute, Wilmington, DE, USA; 2Department of Pain Medicine, NXTSTIM Inc, San Diego, CA, USA; 3Centre for Human Psychopharmacology, Swinburne University of Technology, Hawthorn, VIC, Australia; 4Anatomy and Physiology Department, Faculty of Medicine Dentistry and Health Sciences, The University of Melbourne, Parkville, VIC, Australia
Correspondence: Maja Green, Department of Pain Medicine, NXTSTIM Inc, San Diego, CA, USA, Email [email protected]
Abstract: Chronic pain is a complex and multidimensional condition that disrupts both physical function and cognitive processing, creating a bidirectional cycle that amplifies symptom burden and complicates clinical management. Cognitive dysfunction, characterized by deficits in memory, attention, and executive function, further impairs treatment adherence and functional recovery. Conventional pharmacologic therapies frequently fail to address this dual burden and are associated with adverse effects, including dependence and cognitive impairment. Neuromodulation has emerged as a promising nonpharmacologic alternative, capable of modulating neuroplastic, neuroinflammatory, and neurotransmitter pathways implicated in both pain and cognitive decline. This narrative review examines the mechanisms and clinical applications of spinal cord stimulation (SCS), transcutaneous electrical nerve stimulation (TENS), and neuromuscular electrical stimulation (NMES), and evaluates emerging innovations such as EcoAI™, an artificial intelligence–driven, non-invasive neuromodulation platform. By integrating physiological and behavioral biomarkers with real-time adaptive therapy, EcoAI and similar technologies represent a shift toward personalized, precision-based interventions. Additional advances in remote patient monitoring (RPM) and closed-loop feedback systems further enhance therapeutic responsiveness and continuity of care. Collectively, these approaches offer a scalable, patient-centered framework for managing chronic pain and its cognitive comorbidities. Future priorities include the development of validated biomarkers, rigorous clinical evaluation of AI-integrated systems, and equitable implementation strategies to ensure broad access to next-generation neuromodulation.
Keywords: chronic pain, neuromodulation, cognitive dysfunction, neuroinflammation
Introduction
Chronic pain is a pervasive and multifaceted condition, affecting an estimated 20–30% of the adult population, with prevalence increasing significantly among individuals aged 65 years and older. It imposes substantial physical, psychological and socioeconomic burdens, contributing to functional limitations, reduced quality of life and increased healthcare costs.1–4 Beyond its somatic effects, chronic pain is increasingly recognized as a driver of cognitive dysfunction, with deficits in memory, attention, executive function and processing speed exacerbating disability, particularly in older adults.1,5–7 This bidirectional relationship between pain and cognition forms a self-perpetuating cycle, wherein chronic pain worsens cognitive performance and cognitive dysfunction amplifies pain perception. Standard treatment options predominantly rely on pharmacologic agents such as opioids, nonsteroidal anti-inflammatory drugs (NSAIDs), and adjuvant therapies including antidepressants and anticonvulsants. While these medications may provide symptomatic relief, they also carry notable risks: opioids are associated with tolerance, dependence, and cognitive decline; NSAIDs may lead to gastrointestinal, cardiovascular, or renal complications; and adjuvant agents often cause sedation, dizziness, and further cognitive disruption, limiting daily function.8 As an alternative, neuromodulation has emerged as a promising non-pharmacologic strategy. It acts directly on nociceptive pathways and modulates neuroplasticity and neuroinflammatory signaling involved in both pain and cognitive dysfunction.8–10 Among the leading modalities, spinal cord stimulation (SCS), transcutaneous electrical nerve stimulation (TENS), and neuromuscular electrical stimulation (NMES) offer complementary mechanisms of action and clinical utility. SCS is a well-established invasive technique that inhibits pain transmission through dorsal column modulation while restoring disrupted cortical connectivity. TENS, a non-invasive approach, activates Aβ fibers to reduce nociceptive signaling via spinal gating, opioid-mediated analgesia, and descending inhibition. NMES, while traditionally used for muscle rehabilitation, also engages motor pathways to improve circulation, enhance proprioceptive signaling, and promote neuroplasticity, including the upregulation of brain-derived neurotrophic factor (BDNF). These effects suggest that TENS and NMES may not only relieve pain but also help restore neurocognitive integration in patients affected by chronic pain.11,12
Despite these advancements, challenges remain. Long-term opioid use can negatively impact neuroplasticity, further entrenching the pain-cognition cycle. While invasive neuromodulation is effective, it is costly and carries procedural risks. Non-invasive options like TENS and NMES, although more accessible, require individualized protocols and more robust clinical validation. Large-scale, high-quality trials are still needed to determine the long-term safety, efficacy, and optimal application of these approaches.13 Recent technological innovations, particularly the integration of AI, RPM and remote patient management, offer a transformative opportunity for chronic pain care. AI-driven platforms such as EcoAI, incorporate functional imaging and biomarker analysis to guide personalized neuromodulation in real time. This approach enhances therapeutic precision and may be especially beneficial in patients experiencing concurrent sensory and cognitive dysfunction.
This narrative review critically examines the role of neuromodulation and AI-enabled technologies in chronic pain management, with specific attention to SCS, TENS and NMES. We explore their mechanisms of action, effects on pain and cognitive function, and the evolving landscape of technological innovation. Finally, we highlight the potential of AI-integrated tools like EcoAI to bridge the gap between symptom control and neurocognitive recovery, and outline future directions emphasizing multimodal, patient-centered strategies that combine neuromodulation with cognitive rehabilitation and psychosocial care to improve long-term outcomes in chronic pain.
Chronic Pain and Cognitive Dysfunction: Interconnected Mechanisms and Implications
Chronic pain and cognitive dysfunction are closely linked through a complex interplay of neuroplasticity, neuroinflammation, and neurotransmitter dysregulation. This bidirectional relationship is rooted in the overlapping neural circuits and molecular pathways that govern both nociceptive processing and cognitive functions such as attention, memory, and emotional regulation.14,15
Cognitive impairment in chronic pain originates from maladaptive neuroplastic changes in key brain regions, including the prefrontal cortex, anterior cingulate cortex (ACC), hippocampus and amygdala. Chronic pain contributes to structural atrophy in these areas, leading to deficits in executive function, memory consolidation, and emotional regulation, while simultaneously amplifying pain perception.16–18 For example, the prefrontal cortex and ACC, regions critical for decision-making and emotional processing, show reduced grey matter volume and increased dendritic complexity, which heightens attentional bias toward pain. Similarly, the hippocampus is particularly vulnerable to excitotoxicity and reduced BDNF signalling, which impair synaptic plasticity and long-term memory formation. At the molecular level, excess glutamate activity induces calcium-mediated neurotoxicity, disrupts long-term potentiation (LTP), and diminishes neurogenesis, further impairing adaptive cognitive function.19–22 Neuroinflammatory mechanisms further compound these disruptions. Activated microglia and astrocytes release pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6, sensitizing neurons in both the spinal cord and brain. These cytokines impair synaptic homeostasis by suppressing BDNF production and altering the glutamate/GABA balance, promoting central sensitization and cognitive decline. Astrocytic release of ATP and reactive oxygen species (ROS) perpetuates neuroinflammation and exacerbates dysfunction in regions like the ACC and prefrontal cortex.23–25
Functional imaging studies support these molecular findings. Altered connectivity within the default mode network (DMN) demonstrates increased coupling between pain-processing regions, such as the insula and ACC, alongside reduced engagement of prefrontal cortical areas. These patterns are associated with attentional deficits and increased pain perception.21 At the neurochemical level, disruptions in dopaminergic and serotonergic pathways further undermine cognitive and affective regulation. Reduced dopamine activity in the nucleus accumbens and ventral tegmental area is linked to anhedonia and diminished reward processing, while serotonin depletion exacerbates emotional dysregulation and pain amplification26–28 (Figure 1).
In addition to supraspinal effects, chronic pain also induces maladaptive changes in spinal nociceptive processing. Dorsal horn neurons undergo phenotypic switching, increasing the expression of pro-nociceptive neuropeptides like substance P and CGRP. Simultaneously, GABAergic interneurons lose inhibitory function, fostering hyperexcitability of spinal circuits. This spinal sensitization feeds into supraspinal networks, reinforcing the chronic pain–cognition feedback loop.29–31 Given this complexity, addressing both the physical and cognitive dimensions of chronic pain requires innovative strategies. Neuromodulation techniques, such as SCS, TENS and NMES, target key neurophysiological pathways, including those regulating BDNF expression, neurotransmitter balance and synaptic plasticity.32–42 These approaches not only alleviate nociceptive input but may also reverse cognitive impairment associated with chronic pain. Advancements in functional imaging and AI-driven closed-loop systems further enable real-time personalization of therapy, increasing treatment precision.43–48 By bridging symptom management with cognitive rehabilitation, these integrated approaches offer the potential to disrupt the pathological cycle of pain and cognitive decline. A deeper understanding of the shared and distinct mechanisms underlying chronic pain and neurocognitive dysfunction will inform the development of targeted, multimodal interventions that improve long-term functional outcomes and quality of life.
Neuromodulation Techniques
Neuromodulation has become a cornerstone in the management of chronic pain, evolving from a strategy focused solely on nociceptive inhibition to one that also targets the cognitive dysfunction often associated with chronic pain syndromes. Among these techniques, SCS remains the most established modality, with technological advancements enabling more targeted and individualized treatments that address both sensory and cognitive components of pain.49–51
SCS: Mechanisms and Modalities
Traditional tonic SCS delivers low-frequency stimulation (40–60 hz) to the dorsal columns, aligning with Melzack and Wall’s gate control theory. This approach activates large-diameter Aβ fibers, which inhibit nociceptive C-fiber input via inhibitory interneurons in the spinal cord.52 Although effective for pain relief, tonic SCS has limited impact on supraspinal networks involved in cognition. High-frequency SCS (>1 kHz) addresses this limitation by eliminating paresthesia and restoring functional connectivity across cortical networks. By modulating hyperactivity in the thalamus and prefrontal cortex and suppressing gamma oscillations, high-frequency SCS enhances default mode network (DMN) activity and reduces central sensitization through anti-inflammatory effects (eg, downregulation of TNF-α and IL-6), while promoting GABAergic signaling to support cognitive recovery8,53 (Figure 2).
Burst SCS, which uses intermittent stimulation patterns to mimic natural neuronal firing, engages both ascending pain and descending modulation circuits. It has demonstrated efficacy not only in reducing pain but also in supporting hippocampal neurogenesis and mitigating memory and learning deficits in chronic pain states.53,54 Closed-loop SCS builds upon this by using real-time evoked compound action potentials (ECAPs) to adjust stimulation dynamically, ensuring consistent engagement of target pathways while minimizing overstimulation.34,55 This adaptive system also stabilizes dopaminergic signaling in mesolimbic circuits, enhancing motivation and reducing anhedonia.56–58 Hybrid SCS systems and multi-site approaches, such as dual targeting of dorsal column and lateral corticospinal tracts, further expand therapeutic reach, enabling modulation of both sensory and cognitive pathways in patients with widespread pain or executive dysfunction.59
Non-Invasive Neuromodulation
Non-invasive neuromodulation techniques such as TENS and NMES provide alternative options for patients who are not candidates for invasive procedures. TENS modulates sensory processing by stimulating Aβ fibers, leading to inhibition of nociceptive transmission via gate control mechanisms and endogenous opioid release. NMES primarily targets motor neurons, eliciting muscle contractions that improve circulation, reduce ischemia, and provide afferent feedback to the central nervous system. These effects promote proprioceptive integration and may enhance cortical plasticity and cognitive function. While clinical outcomes with TENS and NMES can be variable, devices such as EcoAI have demonstrated potential to improve consistency through real-time adaptation of stimulation parameters, including frequency, intensity, and waveform tailoring. This personalization expands the clinical utility of non-invasive neuromodulation and may improve access and adherence across diverse populations.60–62
Other Neuromodulation Approaches
Beyond SCS, several advanced neuromodulation strategies have been developed to target anatomically and functionally distinct pain syndromes. Dorsal root ganglion (DRG) stimulation modulates the cell bodies of primary sensory neurons, providing highly localized relief in focal neuropathic pain conditions such as complex regional pain syndrome (CRPS). DRG stimulation has been associated with greater positional stability and superior outcomes in select populations.63,64
Deep brain stimulation (DBS) is a neurosurgical approach that targets central pain-processing centers such as the thalamus, periaqueductal gray or anterior cingulate cortex. It is typically reserved for patients with refractory pain conditions, including central post-stroke pain or brachial plexus avulsion, and aims to modulate both sensory and affective dimensions of pain.65 Occipital nerve stimulation (ONS) involves the placement of subcutaneous electrodes over the occipital nerves and is primarily used for intractable craniofacial pain, such as chronic migraine, cluster headache, or occipital neuralgia. By modulating trigeminocervical pathways in the upper cervical region, ONS addresses pain regions not typically targeted by standard SCS.66 Peripheral field stimulation (PFS), also referred to as peripheral nerve field stimulation, places leads subcutaneously at or near the site of pain, such as in axial low back pain, to engage segmental gating mechanisms. PFS may be used alone or in combination with SCS to broaden the area of pain coverage, particularly for midline or diffuse pain syndromes.67 Collectively, these techniques expand the therapeutic landscape of neuromodulation. By targeting brain regions involved in emotional regulation, executive function, and pain perception, modalities such as DBS and DRG stimulation not only provide analgesia but may also improve cognitive outcomes in chronic pain populations. Their inclusion underscores the importance of a systems-level approach in treating pain and cognition as interconnected domains.
Technological Innovations in Neuromodulation
The field of neuromodulation has advanced significantly through the integration of AI, ML and RPM systems.68 These technologies enable more dynamic, personalized treatment strategies, particularly in non-invasive therapies such as TENS and NMES. A leading example of this innovation is EcoAI, a platform that integrates TENS and NMES functionalities with AI-driven personalization and remote care capabilities. EcoAI distinguishes itself from traditional devices by continuously adapting stimulation parameters, such as pulse frequency, amplitude and duration, in real time based on individual patient profiles. The system interprets both subjective pain scores and objective physiological indicators, including heart rate variability (HRV) and muscle activity, to fine-tune neuromodulation therapy with greater precision. These data-driven adjustments follow a structured, algorithmic model rather than relying on patient-led trial-and-error, ensuring consistent and responsive therapy delivery. With frequency ranges extending up to 1200 hz and expanded waveform versatility, EcoAI offers broader therapeutic targeting than conventional devices.
By merging the analgesic effects of TENS (eg, gate control, endogenous opioid release, and descending inhibition) with the circulation-enhancing and proprioceptive benefits of NMES, EcoAI enables synergistic outcomes for both pain relief and functional rehabilitation. Furthermore, its RPM integration allows clinicians to remotely monitor treatment progress, make adjustments, and identify complications early, expanding access to high-quality care beyond traditional clinical settings.13 This fusion of AI, ML and wearable neuromodulation represents a paradigm shift in digital pain therapy. A recent review by Patel et al compares TENS, EMS and EcoAI across stimulation mechanisms, clinical indications and implementation strategies. The findings underscore EcoAI’s ability to leverage foundational principles from legacy modalities while delivering adaptive, user-specific treatment. Its flexibility, real-time optimization, and compatibility with remote monitoring platforms offer significant advantages in streamlining therapy and addressing both pain and functional deficits.69 Collectively, these features position EcoAI as a next-generation tool in chronic pain management, offering a scalable, intelligent, and patient-centered solution that aligns with the evolving demands of personalized medicine.
Cognitive and Psychomotor Deficits in Chronic Pain: Challenges and Emerging Solutions
Chronic pain exerts profound effects on both cognitive and motor function. Numerous studies have documented consistent impairments in psychomotor speed, perceptual-motor integration, and information processing, with slower reaction times and diminished coordination frequently observed in standardized cognitive testing. These deficits are particularly pronounced in conditions such as diabetic neuropathy, where psychomotor dysfunction is prevalent, yet they remain under-investigated in other chronic pain syndromes. Impaired perceptual learning further compounds these challenges, reducing an individual’s capacity to adapt to external stimuli and altering their ability to respond effectively to their environment.70–72 Beyond motor deficits, cognitive impairments are also a defining feature of chronic pain. Patients commonly report difficulties with concentration, memory and learning, particularly working memory, which supports the temporary storage and manipulation of information. Objective assessments reinforce these complaints, revealing poor performance in spatial and verbal working memory, recognition, and long-term memory tasks.73 These disruptions in executive function often translate into reduced psychosocial performance, impaired workplace productivity, and difficulties with sustained attention.74,75 Mechanistically, these cognitive disturbances are closely tied to chronic pain-induced neuroplastic and neuroinflammatory changes. Structural and functional alterations within the prefrontal cortex, anterior cingulate cortex, and hippocampus undermine higher-order cognitive processes, while elevated levels of pro-inflammatory cytokines such as TNF-α and IL-6, disrupt synaptic signaling and impair neurogenesis. This neuroinflammatory milieu contributes to a vicious cycle in which pain exacerbates cognitive dysfunction, and impaired cognition heightens pain sensitivity and emotional distress.7,76,77 Emerging neuromodulation technologies offer promising solutions to address these intertwined challenges. Advanced platforms are now capable of monitoring cognitive performance indicators, such as working memory load and learning efficiency, in real time. These data can be integrated into adaptive therapeutic algorithms that dynamically tailor stimulation protocols based on patient-specific cognitive and physiological metrics. In addition, combining objective digital biomarkers with patient-reported outcomes helps reconcile subjective experiences with measurable clinical endpoints, improving both diagnostic precision and treatment responsiveness.
As the field continues to evolve, such technologies represent a paradigm shift in chronic pain management. By targeting both the sensory and cognitive dimensions of pain, they enable a more holistic and individualized approach, one that aims not only to reduce pain intensity but also to restore cognitive resilience, functional capacity, and overall quality of life.
Discussion
Chronic pain is a multifaceted condition that disrupts both physical and cognitive function, creating a self-perpetuating cycle that diminishes quality of life and complicates treatment. Cognitive impairments, such as deficits in memory, attention, and executive function, exacerbate these challenges by undermining treatment adherence and limiting participation in rehabilitation. These deficits stem from structural and functional alterations in higher-order brain regions, including the prefrontal cortex and insular cortex, which impair endogenous pain modulation and amplify nociceptive signaling. Addressing this complex pain–cognition interface requires integrative treatment strategies capable of targeting both dimensions. Emerging neuromodulation techniques such as TENS, NMES and EcoAI offer promising solutions. These approaches go beyond nociceptive inhibition by modulating inflammatory cytokines (eg, TNF-α, IL-6) and restoring inhibitory neurotransmission, particularly GABAergic tone, critical for reversing central sensitization and promoting synaptic plasticity.
TENS and NMES contribute to neuromodulation not only by reducing pain perception but also by influencing neuroplasticity and neurotransmitter systems. TENS has been shown to increase endogenous opioid release and promote serotonergic and noradrenergic descending inhibition, helping to reestablish normal pain modulation. NMES facilitates proprioceptive signaling, enhances BDNF production, and improves motor-cognitive integration through repetitive afferent input to the sensorimotor cortex. EcoAI, in particular, represents a paradigm shift in neuromodulation. By combining AI-driven analytics with wearable stimulation and functional imaging, EcoAI enables real-time identification of biomarkers and dynamically adjusts therapy based on individualized neurophysiological profiles. This closed-loop system supports tailored neuromodulation for both sensory and cognitive dysfunction. Figure 3 illustrates EcoAI’s architecture: (A) wearable stimulation targets peripheral nerves; (B) afferent signals and physiological biomarkers are captured; (C) AI algorithms analyze real-time input to adjust stimulation parameters; and (D) clinician dashboards provide remote monitoring of patient progress. This adaptive framework enables precision neuromodulation and facilitates long-term engagement with therapy.
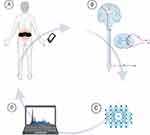 |
Figure 3 EcoAI closed-loop neuromodulation loop. (A) Wearable stimulation device delivers targeted electrical pulses to modulate peripheral nerves; (B) the induced neural pathway responses (eg changes in brain activity or neurotransmitter levels) are captured via integrated sensors; (C) an AI-driven decision-making module analyzes these real-time data streams and dynamically adjusts stimulation parameters; (D) data visualization dashboards display feedback on patient status and therapy efficacy to clinicians. This closed-loop system continuously integrates patient-specific neurophysiological data with adaptive stimulation, enabling real-time monitoring and individualized therapy adjustments for optimized pain and cognitive dysfunction management (Created in BioRender. Green, M. (2025) https://BioRender.com/59e0emj). |
Despite these advances, several challenges remain. Patient responses vary widely, infrastructure to support advanced technology is not universally available, and treatment costs remain a barrier. Data privacy concerns, particularly with AI-integrated systems, also warrant attention. Furthermore, the lack of long-term, large-scale trials evaluating cognitive outcomes limits widespread clinical adoption.
Future research should prioritize the development of validated biomarkers for pain and cognitive dysfunction, enabling precise patient stratification and improving therapeutic consistency. Integrating EcoAI with high-resolution imaging modalities, such as fMRI, may uncover additional mechanisms of action and guide refinement of stimulation protocols. Enhancing the scalability of wearable and AI-integrated platforms will expand access, especially when paired with RPM systems that provide continuous feedback and allow dynamic, real-time intervention. Beyond the biological dimension, integrating cognitive rehabilitation (eg, gamified neurofeedback or virtual reality-based therapies) with neuromodulation may yield synergistic effects. Combining these approaches with psychosocial interventions, pharmacologic therapies and physical rehabilitation offers a robust, multidisciplinary framework for managing chronic pain. To achieve equitable outcomes, implementation must be supported by subsidized programs, expanded insurance coverage, and telemedicine infrastructure.
By uniting patient-centered strategies with personalized technologies, chronic pain management can move beyond symptom suppression to restore cognitive resilience, emotional stability, and functional independence. EcoAI and similar platforms, when deployed within this comprehensive framework, have the potential to fundamentally transform outcomes for individuals living with chronic pain.
Conclusion
Chronic pain is a complex, multidimensional condition that disrupts both physical functionality and cognitive processes, creating a reinforcing cycle of impairment that diminishes quality of life. The bidirectional relationship between pain and cognition complicates treatment: memory, attention, and executive dysfunction hinder therapy adherence and engagement, while untreated pain further degrades cognitive performance. Pharmacologic options, though widely used, are often associated with dependency, side effects, and limited efficacy on cognitive symptoms. Invasive neuromodulation techniques, particularly SCS, remain highly effective for conditions such as post-surgical pain syndrome (PSPS/FBSS) and complex regional pain syndrome (CRPS), supported by robust evidence. However, accessibility and procedural risks limit their widespread use. Non-invasive approaches, including TENS, NMES, and AI-enabled systems like EcoAI, have emerged as compelling alternatives that address both nociceptive and neurocognitive dysfunction by targeting inflammation, neurotransmission, and cortical plasticity.
EcoAI represents a next-generation solution by integrating wearable neuromodulation with real-time data analytics and functional imaging. It personalizes stimulation protocols using patient-specific biomarkers and AI algorithms, delivering responsive, individualized therapy. While promising, these innovations must be evaluated in long-term, multicenter trials to assess durability, safety, and real-world applicability. Ethical considerations, including data privacy and cost barriers, must also be addressed. It is essential to view non-invasive and invasive neuromodulation not as competing approaches but as components of a stratified care model. TENS, NMES, and EcoAI offer lower-risk, accessible options that may complement or precede more invasive interventions. Treatment selection should reflect individual patient needs, clinical context, and therapeutic goals.
Moving forward, chronic pain management must prioritize the development of predictive biomarkers, integration of cognitive rehabilitation and psychosocial strategies, and equitable access to emerging technologies. By embracing multidisciplinary, personalized care, clinicians can move beyond symptom control toward restoring cognition, emotional well-being, and meaningful functionality. This paradigm shift offers renewed hope for patients seeking long-term relief and improved quality of life.
Disclosure
Maja Green, Vida Nazemian, Adam Cabble and Krishnan Chakravarthy are employees of NXTSTIM Inc. Amie Hayley reports grants from National Health and Medical Research Council (NHMRC), outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Higgins DM, Martin AM, Baker DG, Vasterling JJ, Risbrough V. The relationship between chronic pain and neurocognitive function: a systematic review. Clin J Pain. 2018;34(3):262–275. doi:10.1097/AJP.0000000000000536
2. Bouhassira D, Lanteri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136(3):380–387. doi:10.1016/j.pain.2007.08.013
3. Mills SEE, Nicolson KP, Smith BH. Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth. 2019;123(2):e273–e83.
4. Sudak HS. Predicting suicide rates in the elderly. Am J Psychiatry. 2010;167(1):102. doi:10.1176/appi.ajp.2009.09081206
5. Berryman C, Stanton TR, Jane Bowering K, Tabor A, McFarlane A, Lorimer Moseley G. Evidence for working memory deficits in chronic pain: a systematic review and meta-analysis. Pain. 2013;154(8):1181–1196. doi:10.1016/j.pain.2013.03.002
6. Berryman C, Stanton TR, Bowering KJ, Tabor A, McFarlane A, Moseley GL. Do people with chronic pain have impaired executive function? A meta-analytical review. Clin Psychol Rev. 2014;34(7):563–579. doi:10.1016/j.cpr.2014.08.003
7. Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol. 2011;93(3):385–404. doi:10.1016/j.pneurobio.2011.01.002
8. Zhou M, Zhong H, Xing C, et al. Comparison of clinical outcomes associated with spinal cord stimulation (SCS) or conventional medical management (CMM) for chronic pain: a systematic review and meta-analysis. Eur Spine J. 2023;32(6):2029–2041. doi:10.1007/s00586-023-07716-2
9. Malik RN, Samejima S, Shackleton C, et al. REPORT-SCS: minimum reporting standards for spinal cord stimulation studies in spinal cord injury. J Neural Eng. 2024;21(1):016019. doi:10.1088/1741-2552/ad2290
10. Simopoulos TT, Gill JS. Magnetic resonance imaging of the lumbar spine in a patient with a spinal cord stimulator. Pain Physician. 2013;16(3):E295–300. doi:10.36076/ppj.2013/16/E295
11. Johnson MI, Claydon LS, Herbison GP, Jones G, Paley CA. Transcutaneous electrical nerve stimulation (TENS) for fibromyalgia in adults. Cochrane Database Syst Rev. 2017;10(10):CD012172. doi:10.1002/14651858.CD012172.pub2
12. Gibson W, Wand BM, Meads C, Catley MJ, O’Connell NE. Transcutaneous electrical nerve stimulation (TENS) for chronic pain - an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2019;4(4):CD011890. doi:10.1002/14651858.CD011890.pub3
13. Patel PM, Green M, Tram J, et al. Beyond the pain management clinic: the role of AI-integrated remote patient monitoring in chronic disease management - a narrative review. J Pain Res. 2024;17:4223–4237. doi:10.2147/JPR.S494238
14. Hatzis A, Stranjalis G, Megapanos C, Sdrolias PG, Panourias IG, Sakas DE. The current range of neuromodulatory devices and related technologies. Acta Neurochir Suppl. 2007;97(Pt 1):21–29. doi:10.1007/978-3-211-33079-1_3
15. Crunkhorn S. Developing closed-loop neuromodulatory devices. Nat Rev Drug Discov. 2019;18(2):98.
16. Loeser JD, Melzack R. Pain: an overview. Lancet. 1999;353(9164):1607–1609. doi:10.1016/S0140-6736(99)01311-2
17. Tyng CM, Amin HU, Saad MNM, Malik AS. The influences of emotion on learning and memory. Front Psychol. 2017;8. doi:10.3389/fpsyg.2017.01454
18. Phelps CE, Navratilova E, Porreca F. Cognition in the chronic pain experience: preclinical insights. Trends Cogn Sci. 2021;25(5):365–376. doi:10.1016/j.tics.2021.01.001
19. Pask S, Dell’Olio M, Murtagh FEM, Boland JW. The effects of opioids on cognition in older adults with cancer and chronic noncancer pain: a systematic review. J Pain Symptom Manage. 2020;59(4):871–93e1. doi:10.1016/j.jpainsymman.2019.10.022
20. Khera T, Rangasamy V. Cognition and pain: a review. Front Psychol. 2021;12:673962. doi:10.3389/fpsyg.2021.673962
21. Malfliet A, Coppieters I, Van Wilgen P, et al. Brain changes associated with cognitive and emotional factors in chronic pain: a systematic review. Eur J Pain. 2017;21(5):769–786. doi:10.1002/ejp.1003
22. Treede R-D, Rief W, Barke A, et al. A classification of chronic pain for ICD-11. Pain. 2015;156(6):1003. doi:10.1097/j.pain.0000000000000160
23. Feldman RA. Microglia orchestrate neuroinflammation. Elife. 2022;11. doi:10.7554/eLife.81890
24. Woodburn SC, Bollinger JL, Wohleb ES. The semantics of microglia activation: neuroinflammation, homeostasis, and stress. J Neuroinflammation. 2021;18(1):258. doi:10.1186/s12974-021-02309-6
25. Wang C, Zong S, Cui X, et al. The effects of microglia-associated neuroinflammation on Alzheimer’s disease. Front Immunol. 2023;14:1117172. doi:10.3389/fimmu.2023.1117172
26. Labrakakis C. The Role of the Insular Cortex in Pain. Int J Mol Sci. 2023;24(6):5736. doi:10.3390/ijms24065736
27. Fan N, Chen J, Zhao B, et al. Neural correlates of central pain sensitization in chronic low back pain: a resting-state fMRI study. Neuroradiology. 2023;65(12):1767–1776. doi:10.1007/s00234-023-03237-3
28. Li T, Zhang S, Ikeda E, Kobinata H. Functional connectivity modulations during offset analgesia in chronic pain patients: an fMRI study. Brain Imaging Behav. 2022;16(4):1794–1802. doi:10.1007/s11682-022-00652-7
29. Taulaniemi A, Kankaanpaa M, Rinne M, Tokola K, Parkkari J, Suni JH. Fear-avoidance beliefs are associated with exercise adherence: secondary analysis of a randomised controlled trial (RCT) among female healthcare workers with recurrent low back pain. BMC Sports Sci Med Rehabil. 2020;12:28. doi:10.1186/s13102-020-00177-w
30. Osuka S, Koshino Y, Watanabe K, Kataoka Y, Tohyama H. Fear-avoidance beliefs associated with non-specific chronic low back pain in college athletes. J Pain Res. 2024;17:285–292. doi:10.2147/JPR.S447121
31. Delpierre Y. Fear-avoidance beliefs, anxiety and depression are associated with motor control and dynamics parameters in patients with chronic low back pain. J Orthop. 2022;29:44–49. doi:10.1016/j.jor.2022.01.005
32. Hart RP, Wade JB, Martelli MF. Cognitive impairment in patients with chronic pain: the significance of stress. Curr Pain Headache Rep. 2003;7(2):116–126. doi:10.1007/s11916-003-0021-5
33. Stauss T, El Majdoub F, Sayed D, et al. A multicenter real-world review of 10 kHz SCS outcomes for treatment of chronic trunk and/or limb pain. Ann Clin Transl Neurol. 2019;6(3):496–507. doi:10.1002/acn3.720
34. Mekhail NA, Levy RM, Deer TR, et al. ECAP-controlled closed-loop versus open-loop SCS for the treatment of chronic pain: 36-month results of the EVOKE blinded randomized clinical trial. Reg Anesth Pain Med. 2024;49(5):346–354. doi:10.1136/rapm-2023-104751
35. Kapural L, Wu C, Calodney A, et al. Demographics and PainDETECT as predictors of 24-month outcomes for 10 kHz SCS in nonsurgical refractory back pain. Pain Physician. 2024;27(3):129–139. doi:10.36076/ppj.2024.7.129
36. Kapural L, Calodney A. Retrospective efficacy and cost-containment assessment of 10 kHz Spinal Cord Stimulation (SCS) in non-surgical refractory back pain patients. J Pain Res. 2022;15:3589–3595. doi:10.2147/JPR.S373873
37. McCracken LM, Gross RT, Sorg P, Edmands TA. Prediction of pain in patients with chronic low back pain: effects of inaccurate prediction and pain-related anxiety. Behav Res Ther. 1993;31(7):647–652. doi:10.1016/0005-7967(93)90117-D
38. Saraogi M, Geraghty RM, Hameed B, et al. Role of complementary medicine (Music, Acupuncture, Acupressure, TENS and Audio-Visual Distraction) in Shockwave Lithotripsy (SWL): a systematic review from EAU Sections of Urolithiasis (EULIS) and Uro-Technology (ESUT). Urology. 2020;145:38–51. doi:10.1016/j.urology.2020.06.035
39. Johnson MI, Paley CA, Jones G, Mulvey MR, Wittkopf PG. Efficacy and safety of transcutaneous electrical nerve stimulation (TENS) for acute and chronic pain in adults: a systematic review and meta-analysis of 381 studies (the meta-TENS study). BMJ Open. 2022;12(2):e051073. doi:10.1136/bmjopen-2021-051073
40. Cheema AS, Doyon J, Lapner P. Transcutaneous electrical nerve stimulation (TENS) and extracorporeal shockwave therapy (ESWT) in lateral epicondylitis: a systematic review and meta-analysis. JSES Int. 2023;7(2):351–356. doi:10.1016/j.jseint.2022.11.002
41. Arik MI, Kiloatar H, Aslan B, Icelli M. The effect of TENS for pain relief in women with primary dysmenorrhea: a systematic review and meta-analysis. Explore. 2022;18(1):108–113. doi:10.1016/j.explore.2020.08.005
42. Arienti C. Is transcutaneous electrical nerve stimulation (TENS) effective in adults with fibromyalgia? A Cochrane Review summary with commentary. J Musculoskelet Neuronal Interact. 2019;19(3):250–252. doi:10.1002/14651858.CD012172.pub2/full
43. Dufton BD. Cognitive failure and chronic pain. Int J Psychiatry Med. 1989;19(3):291–297. doi:10.2190/JDJK-0795-5BFL-5N6K
44. Jamison RN, Matt DA, Parris WC. Treatment outcome in low back pain patients: do compensation benefits make a difference? Orthop Rev. 1988;17(12):1210–1215.
45. Kewman DG, Vaishampayan N, Zald D, Han B. Cognitive impairment in musculoskeletal pain patients. Int J Psychiatry Med. 1991;21(3):253–262. doi:10.2190/FRYK-TMGA-AULW-BM5G
46. Alanoglu E, Ulas UH, Ozdag F, Odabasi Z, Cakci A, Vural O. Auditory event-related brain potentials in fibromyalgia syndrome. Rheumatol Int. 2005;25(5):345–349. doi:10.1007/s00296-004-0443-3
47. Bosma H, van Boxtel MP, Ponds RW, et al. Engaged lifestyle and cognitive function in middle and old-aged, non-demented persons: a reciprocal association? Z Gerontol Geriatr. 2002;35(6):575–581. doi:10.1007/s00391-002-0080-y
48. Legrain V, Damme SV, Eccleston C, Davis KD, Seminowicz DA, Crombez G. A neurocognitive model of attention to pain: behavioral and neuroimaging evidence. Pain. 2009;144(3):230–232. doi:10.1016/j.pain.2009.03.020
49. Sdrulla AD, Guan Y, Raja SN. Spinal cord stimulation: clinical efficacy and potential mechanisms. Pain Pract. 2018;18(8):1048–1067. doi:10.1111/papr.12692
50. Streumer J, Selvaraj AK, Kurt E, et al. Does spinal cord stimulation improve gait in Parkinson’s disease: a comprehensive review. Parkinsonism Relat Disord. 2023;109:105331. doi:10.1016/j.parkreldis.2023.105331
51. Garcia MA, Emami AS, Blau LE, Rutledge T. A pre- and post-implantable pain device procedure assessment model: psychiatric symptoms, functioning, and goals. Pain Manag. 2023;13(3):161–170. doi:10.2217/pmt-2022-0087
52. Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971–979. doi:10.1126/science.150.3699.971
53. Meuwissen KPV, Gu JW, Zhang TC, Joosten EAJ. Conventional-SCS vs. Burst-SCS and the behavioral effect on mechanical hypersensitivity in a rat model of chronic neuropathic pain: effect of amplitude. Neuromodulation. 2018;21(1):19–30. doi:10.1111/ner.12731
54. Vesper J, Slotty P, Schu S, et al. Burst SCS microdosing is as efficacious as standard burst SCS in treating chronic back and leg pain: results from a randomized controlled trial. Neuromodulation. 2019;22(2):190–193. doi:10.1111/ner.12883
55. Nijhuis H, Kallewaard JW, van de Minkelis J, et al. Durability of Evoked Compound Action Potential (ECAP)-controlled, closed-loop Spinal Cord Stimulation (SCS) in a real-world European chronic pain population. Pain Ther. 2024;13(5):1119–1136. doi:10.1007/s40122-024-00628-z
56. Schieferdecker S, Neudorfer C, El Majdoub F, Maarouf M. A retrospective case series of high-frequency spinal cord stimulation (HF10-SCS) in neurogenic bladder incontinence. Oper Neurosurg. 2019;17(1):14–20. doi:10.1093/ons/opy236
57. Russo M, Van Buyten JP. 10-kHz high-frequency SCS therapy: a clinical summary. Pain Med. 2015;16(5):934–942. doi:10.1111/pme.12617
58. Provenzano DA, Park N, Edgar D, Bovinet C, Tate J. High-frequency (10 kHz) spinal cord stimulation (SCS) as a salvage therapy for failed traditional SCS: a narrative review of the available evidence. Pain Pract. 2023;23(3):301–312. doi:10.1111/papr.13184
59. Upreti P, Ojha CSP. Development and performance evaluation of SCS-CN based hybrid model. Water Sci Technol. 2022;85(9):2479–2502. doi:10.2166/wst.2022.145
60. Kroeling P, Gross A, Graham N, et al. Electrotherapy for neck pain. Cochrane Database Syst Rev. 2013;2013(8):Cd004251. doi:10.1002/14651858.CD004251.pub5
61. Müller T. Anti-inflammatory and analgesic electrotherapy. Evidence in rheumatology? Z Rheumatol. 2009;68(7):530,2–3.
62. Burcea CC, Oancea MD, Tache-Codreanu DL, Georgescu L, Neagoe IC, Sporea C. The benefits of a rehabilitation program following medial patellofemoral ligament reconstruction. Life. 2024;14(11):1355. doi:10.3390/life14111355
63. Hunter CW, Sayed D, Lubenow T, et al. DRG FOCUS: a multicenter study evaluating dorsal root ganglion stimulation and predictors for trial success. Neuromodulation. 2019;22(1):61–79. doi:10.1111/ner.12796
64. Chapman KB, Sayed D, Lamer T, et al. Best practices for dorsal root ganglion stimulation for chronic pain: guidelines from the American Society of Pain and Neuroscience. J Pain Res. 2023;16:839–879. doi:10.2147/JPR.S364370
65. Farrell SM, Green A, Aziz T. The current state of deep brain stimulation for chronic pain and its context in other forms of neuromodulation. Brain Sci. 2018;8(8):158. doi:10.3390/brainsci8080158
66. Sakharpe AK, Cascella M. Occipital Nerve Stimulation. Treasure Island (FL): StatPearls; 2025.
67. Reverberi C, Dario A, Barolat G. Spinal cord stimulation (SCS) in conjunction with peripheral nerve field stimulation (PNfS) for the treatment of complex pain in failed back surgery syndrome (FBSS). Neuromodulation. 2013;16(1):
68. Tan SY, Sumner J, Wang Y, Wenjun Yip A. A systematic review of the impacts of remote patient monitoring (RPM) interventions on safety, adherence, quality-of-life and cost-related outcomes. NPJ Digit Med. 2024;7(1):192. doi:10.1038/s41746-024-01182-w
69. Patel P, Green M, Tram J, et al. Latest advancements in Transcutaneous Electrical Nerve Stimulation (TENS) and Electronic Muscle Stimulation (EMS): revisiting an established therapy with new possibilities. J Pain Res. 2025;18:137–153. doi:10.2147/JPR.S493162
70. Maihofner C, DeCol R. Decreased perceptual learning ability in complex regional pain syndrome. Eur J Pain. 2007;11(8):903–909. doi:10.1016/j.ejpain.2007.03.006
71. Rahimzadeh S, Ghanavati T, Pourreza S, et al. Inter-joint coordination during obstacle crossing in people with diabetic neuropathy. J Biomech. 2020;105:109765. doi:10.1016/j.jbiomech.2020.109765
72. Glass JM, Park DC, Minear M, Crofford LJ. Memory beliefs and function in fibromyalgia patients. J Psychosom Res. 2005;58(3):263–269. doi:10.1016/j.jpsychores.2004.09.004
73. Rosner M, Sabo M, Klatt LI, Wascher E, Schneider D. Preparing for the unknown: how working memory provides a link between perception and anticipated action. Neuroimage. 2022;260:119466. doi:10.1016/j.neuroimage.2022.119466
74. Luerding R, Weigand T, Bogdahn U, Schmidt-Wilcke T. Working memory performance is correlated with local brain morphology in the medial frontal and anterior cingulate cortex in fibromyalgia patients: structural correlates of pain-cognition interaction. Brain. 2008;131(Pt 12):3222–3231. doi:10.1093/brain/awn229
75. Oosterman JM, Dijkerman HC, Kessels RP, Scherder EJ. A unique association between cognitive inhibition and pain sensitivity in healthy participants. Eur J Pain. 2010;14(10):1046–1050. doi:10.1016/j.ejpain.2010.04.004
76. Jamison RN, Sbrocco T, Parris WC. The influence of problems with concentration and memory on emotional distress and daily activities in chronic pain patients. Int J Psychiatry Med. 1988;18(2):183–191.
77. Munoz M, Esteve R. Reports of memory functioning by patients with chronic pain. Clin J Pain. 2005;21(4):287–291. doi:10.1097/01.ajp.0000173993.53733.2e
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Normalization of Neuroinflammation: A New Strategy for Treatment of Persistent Pain and Memory/Emotional Deficits in Chronic Pain
Liu XG
Journal of Inflammation Research 2022, 15:5201-5233
Published Date: 9 September 2022
Radiofrequency Ablation for Chronic Lumbar Zygapophyseal Joint Pain Using a V-Shaped Active Tip Needle: An Observational Retrospective Study
Lo Bianco G, Misseri G, Stogicza AR, Cesare G, Li S, Day M, Kennedy DJ, Schatman ME
Journal of Pain Research 2023, 16:1243-1255
Published Date: 11 April 2023
Pain and Transcranial Direct Current Stimulation: A Bibliometric Analysis
Chiriac VF, Leucuța DC, Moșoiu DV
Journal of Pain Research 2023, 16:3655-3671
Published Date: 1 November 2023
Spinal Cord Stimulation for Intractable Visceral Pain Originating from the Pelvic and Abdominal Region: A Narrative Review on a Possible New Indication for Patients with Therapy-Resistant Pain
Bieze M, van Haaps AP, Kapural L, Li S, Ferguson K, de Vries R, Schatman ME, Mijatovic V, Kallewaard JW
Journal of Pain Research 2024, 17:691-736
Published Date: 19 February 2024
Identification and Evaluation of Lipocalin-2 in Sepsis-Associated Encephalopathy via Machine Learning Approaches
Hu J, Chen Z, Wang J, Xu A, Sun J, Xiao W, Yang M
Journal of Inflammation Research 2025, 18:3843-3858
Published Date: 14 March 2025



