Back to Journals » Research and Reports in Urology » Volume 17
Transurethral Water Vapor Ablation: Potential for a Novel Prostate Cancer Management Strategy
Authors Warlick CA, Spilseth BD, Dixon CM
Received 1 October 2024
Accepted for publication 22 January 2025
Published 3 February 2025 Volume 2025:17 Pages 17—25
DOI https://doi.org/10.2147/RRU.S498872
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Panagiotis J Vlachostergios
Christopher A Warlick,1,* Benjamin D Spilseth,2,* Christopher M Dixon3,*
1Department of Urology, University of Minnesota, Minneapolis, MN, USA; 2Department of Radiology, University of Minnesota, Minneapolis, MN, USA; 3WMC Health Good Samaritan Hospital, Bon Secours Urology, Suffern, NY, USA
*These authors contributed equally to this work
Correspondence: Christopher A Warlick, University of Minnesota, Department of Urology, 420 Delaware Street SE, B542 Mayo, Minneapolis, MN, 55455, USA, Tel +1-612-625-7486, Email [email protected]
Purpose: Targeted and precise application of thermal energy stored in sterile water vapor is a novel approach to treat cancerous prostate tissue. We report safety and oncological results of transurethral hemigland vapor ablation in a prospective, single-arm study in men with unilateral, intermediate-risk localized prostate cancer.
Patients and Methods: Men with biopsy confirmed Gleason Grade Group 2 (GG2) adenocarcinoma of the prostate, mean age 64.6 years, PSA 5.2 ng/mL, and prostate volume 46.8 cc on TRUS were enrolled. Using cystoscopy and transrectal ultrasound (TRUS) guidance, water vapor (~103°C) was delivered to prostate zones for hemigland ablation inclusive of cancers identified by multiparamertric MRI (mpMRI) and biopsy. At 6-months, combined 12-core systematic and mpMRI fusion biopsy was performed. Subjects with no remaining GG2 disease were followed for 12 months. Those with residual or newly identified GG2 disease were eligible for a second vapor ablation and subsequent 6-month mpMRI fusion biopsy and were followed for at least 18 months after index treatment.
Results: Fifteen subjects were successfully treated. At 6 months 4/15 subjects were identified for further management, two with residual GG2 cancer on the treated side, and two with newly identified GG2 cancer on the untreated contralateral side; one of two subjects with residual GG2 was ineligible for retreatment (unrelated myocardial infarction). Follow up at 12 to 18 months after initial or retreatment provided a final ≥GG2 negative biopsy in 14/15 (93.3%) subjects. Device or procedure-related adverse events (AEs) were mild/moderate and transient; none were serious AEs.
Conclusion: Water vapor ablation has low morbidity. It is possible to successfully retreat residual disease or new lesions identified on surveillance with only transient mild to moderate adverse events and acceptable oncologic outcomes offering a new management strategy for localized prostate cancer.
Plain Language Summary: Treatment decisions for men with localized prostate cancer (PCa) are determined with consideration of multiple factors including, disease risk, co-morbidities, estimated life-expectancy and each patient’s preferences. Currently, many men with low-risk disease are offered active surveillance, during which the prostate cancer is closely monitored for any changes, whereas men with intermediate or high-risk localized cancers are more often considered for surgical removal of the prostate or treatment with radiation. This study was conducted to evaluate a novel therapy designed to destroy PCa tissue using water vapor ablation in men with intermediate-risk PCa confined to one side of the prostate. Dr. Warlick et al used this new technology to treat tumors that were visible under magnetic resonance imaging (MRI) and confirmed to be cancerous via biopsy. The study also includes results from a subset of patients that received additional treatments of newly identified cancer in previously untreated areas on the other side of the prostate, or retreatment of residual (missed) cancer after the initial procedure. Patients experienced no serious side effects after treatment. Results of this feasibility study support further exploration of this new therapeutic procedure and the opportunity it may offer to men to manage their localized prostate cancer through surveillance and additional therapy as needed.
Keywords: prostatic neoplasms, focal ablation, minimally invasive surgical therapy, mpMRI, prospective study
Introduction
Treatment decisions for men with localized prostate cancer (PCa) are navigated with consideration of multiple factors including, disease risk, co-morbidities, estimated life-expectancy and patient preferences. Currently, many men with low-risk disease are offered active surveillance, whereas men with intermediate or high-risk localized cancers are candidates for definitive therapy with radical prostatectomy or radiation (typically with adjuvant hormonal therapy). Partial gland therapy using thermal and non-thermal energy sources has been investigated as an alternative to definitive therapy or active surveillance.1–5 This therapy with water vapor ablation ideally offers elimination of the clinically significant cancerous tissue while sparing uninvolved prostate tissue and surrounding structures and thus likelihood of extended cancer control with less morbidity than associated with definitive therapy.
Thermal water vapor technology uses the energy stored in a few drops of water vapor to ablate prostate tissue. Convectively delivered water vapor (steam) has previously been shown to effectively treat benign prostate hyperplasia with an excellent safety profile.6–8 A first feasibility study (VAPOR 1) used water vapor technology in a partial gland treatment strategy that included unilateral biopsy confirmed visible PCa lesions identified by multiparamertric MRI (mpMRI) in a hemigland protocol.9 The results of the 6-month follow-up study revealed that it is a safe procedure with low short-term morbidity.9
We herein report the final, 12-month safety and oncological results of transurethral hemigland thermal water vapor ablation in our prospective, single-arm study in men with unilateral, intermediate-risk PCa. We also report on the subset of the 15 patients requiring additional treatment for newly identified contralateral prostate cancer (out-of-field) or retreatment of residual in-field cancer. This introduces the paradigm of managing clinically significant localized prostate cancer through surveillance and treatment (with re-treatment) when needed.
Materials and Methods
Study Objectives, Subjects, and Protocol
The primary objective of the VAPOR 1 Study was to assess the safety of water vapor ablation in patients with unilateral, intermediate risk, localized prostate cancer in anticipation of a larger pivotal trial. Secondary objectives included assessment of the effectiveness of vapor ablation in eradicating mpMRI identified, biopsy confirmed cancer anywhere in the prostate and to evaluate the safety and effectiveness of additional retreatment for persistent in-field or newly discovered Gleason Grade Group (GG) 2 disease.
Briefly, 15 men with intermediate risk PCa were treated using transurethral water vapor ablation at four centers in the United States (VAPOR 1 Study; www.ClinicalTrials.gov NCT04087980). Key eligibility criteria included no prior prostate cancer treatment and fusion biopsy-confirmed unilateral, GG2 adenocarcinoma of the prostate, clinical stage ≤T2b, PSA ≤15 ng/mL, a prostate volume 20 to 80 cc as measured by transrectal ultrasound (TRUS), and no evidence of metastases. A complete list of the inclusion and exclusion criteria has previously been reported.9 At 6 months following treatment, subjects underwent systematic and MRI/US fusion biopsy to assess for residual disease.
Patients with residual in field GG2 or newly identified GG2 on the non-treated side were considered for additional treatment. Subjects were eligible for retreatment if they again met the original inclusion criteria. After re-screening, subjects eligible for a second vapor ablation underwent identical follow-up assessment as for the index treatment. Study exit visits occurred 18 to 21 months after the index vapor treatment.
The study protocol was approved by institutional review boards for enrolling sites (University of Minnesota IRB #IORG0000261, New York Medical Center IRB #IORG000513, WCG IRB #IORG0000432); research was conducted in accordance with the Declaration of Helsinki as revised in 2013, and participants gave written informed consent.
Pre-Operative, Interim and Final Study Imaging
Prior to treatment, MRI studies of the prostate were performed (T2-weighted, diffusion-weighted and dynamic gadolinium contrast enhanced images) and assessed using Prostate Imaging Reporting & Data System, (PI-RADS) v2.1.10 Eligibility for treatment or retreatment was confirmed by each site radiologist and a central study radiologist. Planning for treatment, biopsy, and subsequent retreatment involved segmentation of the prostate and delineation of MRI-visible suspicious lesions performed by a single subspecialized radiologist with over 10 years’ experience interpreting prostate MRI. Volume was calculated at all timepoints using the modified ellipsoid formula.
MRIs were subsequently performed 7 days post treatment to assess the extent of ablation and at 6 months to plan the 6-month follow-up biopsy in the treated prostate. At the 6-month post treatment visit, subjects had a combined 12-core systematic and MRI/TRUS fusion biopsy. The sampled cores included systematic cores from the treated side, heightened sampling of any central-reader identified suspicious or indeterminate regions on the treated side, targeted cores from all lesions defined as PI-RADS ≥3 on the untreated, contralateral side, and systematic cores from the untreated side. For subjects with no GG2 cancer found at the 6-month fusion biopsy, an MRI was repeated at the 12-month study exit visit.
MRI evaluations of retreated subjects with residual or new lesions followed an identical pattern to that followed the index treatments including an MRI at 7 days post retreatment and an MRI at 6 months post second treatment which was 18–21 months post index procedure.
Transurethral Vapor Ablation Procedure
Thermal water vapor therapy was delivered using an early version investigational device (Poseidon System, Francis Medical, Inc., Maple Grove, Minnesota, USA). Briefly, the system is comprised of a generator and a transurethral delivery device that contains a deployable, variable length needle controlled by the physician and monitored under real-time transrectal ultrasound. The needle is deployed into the appropriate anatomic zone and location in the prostate and water vapor (103°C) is delivered for 10 seconds resulting in irreversible thermal tissue ablation. The needle can be advanced or retracted at 1mm increments along the same needle path or repositioned for treatment in additional locations within the prostate. In this study, the treatment strategy included individual, overlapping vapor treatments delivered to all regions of the prostate within the transition zone and peripheral zone to treat lesions categorized as PI-RADS ≥3 and to complete the hemiablation. Details of the procedure were provided in the earlier report.9
Safety and Effectiveness Assessments
The primary safety endpoint was frequency of device-related serious adverse events (SAEs) related to rectal fistula, perforation, dissection, urosepsis, significant infection, and de novo serious urinary incontinence. Secondary safety endpoints included potential occurrence of urethral stricture including bladder neck contracture, prolonged urinary retention, delayed or prolonged tissue sloughing requiring instrumentation or endoscopic intervention, and all other reported adverse events (AEs). AEs were assessed at each clinic visit at days 7, 30 and 90, 6 months, and end of study. These were adjudicated by an independent reviewer using Common Terminology Criteria for Adverse Events (CTCAE) v5.0.11
Effectiveness evaluations included negative biopsy on the treated side after index and added or retreatments, assessment for residual suspicious lesions on MRI, reductions in PSA and prostate volume via MRI, and effects on patient reported QOL assessments. A series of standard validated patient-reported questionnaires were administered to assess urinary tract symptoms (IPSS and QOL), sexual function (IIEF, MSHQ-EjD, MSHQ Bother and EPIC Sexual Domain), QOL after treatment for prostate cancer (EPIC 32 Expanded Prostate Cancer Index Composite),12 and Pelvic Pain Score.
Statistical Methods
Descriptive statistics performed with SAS, v9.4 described baseline subject characteristics and endpoints in the study.
Results
The 15 treated subjects had a baseline mean age of 64.6 years [range 49–78], mean PSA of 5.2 ng/mL [1.2 −11.5] and mean prostate volume of 46.8 cc [24.2–77.0] measured by TRUS. A complete list of baseline characteristics was previously presented.9
All 15 patients underwent the protocol required 6-month biopsy and subsequent 12-month follow-up. Thirteen of 15 subjects (86.6%) were biopsy negative at 6-months for ≥GG2 disease on the treated side as previously reported for the interim evaluation.9 Following the 6-month visit, four of 15 subjects were identified for possible further management. Two patients had residual MRI-suspicious appearing areas suggesting partially treated lesions that were ultimately biopsy proven in-field, GG2 cancer. One of these subjects was then ineligible for further treatment due to a myocardial infarction unrelated to the procedure. Two other patients were found to have GG2 disease on the untreated, contralateral side, one through a targeted core and the other through a standard sector core. The three eligible subjects underwent an additional vapor procedure and showed complete ablation of MRI visible lesions on the 7-day and subsequent 6-month MRI. All three 6-month biopsies performed after the second treatments were negative for GG2 disease in-field. One of the three patients had an in-field GG1 core with <5% involvement. The disposition of subjects is shown in Figure 1.
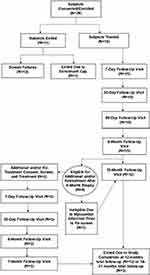 |
Figure 1 Subject flowchart with status of subjects in the index treatment (n=15) and in the additional and /or retreatment cohort (n=3). |
The oncological outcomes in this study through first treatment or retreatment for up to 18 months are summarized in Figure 2. Follow up after completed treatments showed a final ≥GG2 negative biopsy rate of 93.3% (14 of 15) for the entire cohort of subjects with one patient ineligible for retreatment due to an interim myocardial infarction. Figure 3 shows a series of MRI images showing the ablation of a PI-RADS 4 lesion after the index hemigland treatment and subsequent treatment of a newly identified lesion on the untreated, contralateral side of prostate.
Safety Evaluations and Functional Outcomes
All hemigland water vapor ablation procedures were completed as planned. In the previously reported 6-month evaluations the safety profile was unremarkable.9 A total of 16 procedure-related AEs were reported as procedure-related events (11 unique events) in nine subjects, all grade 1 or 2 per the CTCAE classification. AEs through the 12-month study exit (12 subjects) and in the three subjects who underwent an additional, or retreatment are presented in Table 1. There were no added device and/or procedure related adverse events reported in the three subjects after undergoing a second treatment. Overall study safety reporting shows that half of the reported AEs occurring after the index treatment resolved within one month and 75% resolved by three months. At 12 months, the three unresolved procedure-related AEs included one subject with ED (grade 2), one with EjD (grade 2), and one with urinary frequency (grade 1).
 |
Table 1 Procedure-Related Adverse Events Following One or More Vapor Ablation Procedures and Status After First Vapor Procedure (n=15) |
Average days of first catheterization for 15 subjects was 2.9 (range 1–7) with four subjects requiring re-catheterization. Of the four patients re-catheterized, one required catheterization >30 days due to the inability to complete self-intermittent catheterization. There was no long-term chronic retention in the four subjects that were re-catheterized. There was no re-catheterization in the three subjects with repeat or additional treatment. For the three subjects with repeat or additional treatment, average days of catheterization was 3.7 (range 2–5) with no re-catheterization needed in any of the three subjects.
For the 12 subjects receiving a single index procedure, at 12 months the mean PSA reduction of 2.5 ng/mL corresponds to a 57.3 ± 19.2% reduction from the mean baseline value of 5.2 ng/mL (Table 2). The PSA reductions, while suggestive of a surrogate marker of treatment effectiveness, are considered observational due to the wide distribution of the PSA changes. At 6 months after treatment, prostate volume decreased by a mean of 34.5 ±16.6%. Further prostate tissue absorption occurring between the 6- and 12-month study visits resulted in an overall prostate volume reduction of 45.0 ±16.9% (n=12). For three subjects receiving a second treatment, mean PSA and mean prostate volume reductions from baseline to 6-months after the second treatment were a mean of 72.6 ± 19.3% and 51.8 ± 20.0%, respectively.
 |
Table 2 Percent PSA and Prostate Volume Reductions From Baseline After Water Vapor Treatment |
At 12-month follow-up, subject-reported functional and QOL outcomes had no clinically meaningful changes from baseline values at the 6-month9 or 12-month assessments (Supplementary Table).
Discussion
The primary objective of the VAPOR 1 Study was to assess the safety of water vapor ablation in patients with unilateral, intermediate risk, localized prostate cancer in anticipation of a larger pivotal trial. This objective was achieved as there were no serious device related adverse events and those AEs encountered adjudicated as low grade using the CTCAE classification. No intraoperative complications occurred, and the safety profile reflects low levels of adverse events in this limited group of patients. There was no urinary incontinence, rectal injury, or fistula. One patient experienced new onset erectile dysfunction.
This feasibility and safety study has limitations for generalization due to the small sample size and single-arm design. Nevertheless, observations show low morbidity, and that transurethral water vapor technology can ablate intermediate-risk prostate cancer in biopsy confirmed, MRI visible lesions in any region of the prostate. This was suggested by the 7-day contrast enhanced images clearly defining the ablation zone as a distinct region of nonperfusion and confirmed by biopsy in most patients. PSA and prostate volume reduction levels observed were consistent with a hemiablation strategy. It is noteworthy that more PSA and volume reductions occurred beyond 6 months and to at least 12 months. Anatomically, we were able to treat in all areas of the prostate including anteriorly and at the distal apex in the midline. In total, there were 20 lesions targeted for treatment with several located in more than one anatomical region, 12 in apex, 6 in base, and 8 in mid region.9 This is significant because these are traditionally difficult to treat areas for other focal therapy technologies.
The unique aspect of thermal water vapor ablation is convection. Most existing ablative therapies rely on conductive heating or cooling to ablate tissue. Water vapor is fundamentally different as the steam is convectively “blown” into the interstitial spaces between cells. Convection is a much more rapid ablation process as compared to conduction. In addition, it is ideally suited to the anatomic zones of the prostate defined by McNeil.13 Because convection respects the anatomic boundaries of the prostate, including the surgical pseudocapsule and outer prostate capsule, vapor ablation is a zonal treatment and is delivered into the desired anatomical zone, for example the peripheral or transition zone, but not both simultaneously.14
The treatment strategy of this study utilized hemigland ablation to ascertain an initial safety profile of water vapor ablation in the peripheral and transition zones and from apex to base. Despite the low morbidity in this study, full hemigland ablation was likely unnecessary leading to delivery of redundant or overlapping treatments by focusing on the completeness of the hemiablation rather than a more targeted eradication of the known cancer and margin. The focus of future trials will be the index lesion plus an adequate margin with the goal of managing clinically significant cancer over time while maintaining the patient’s quality of life.
Conclusion
Water vapor ablation has low morbidity. It is possible to successfully retreat residual disease or new lesions identified on surveillance with only transient mild to moderate adverse events and acceptable oncologic outcomes. The unique design of this study offers insight into the potential for managing prostate cancer with partial-gland therapy. A high degree of successful eradication of PIRADS > 3 lesions, PSA reduction, and most importantly, negative or <GG1 disease on biopsy suggests the ability to successfully manage intermediate risk prostate cancer with one or more vapor treatments. The avoidance of definitive therapy can be considered a significant benefit of partial gland vapor therapy.15 This strategy may make patients who might not otherwise be considered ideal candidates for active surveillance, subsequently more eligible for ongoing surveillance. Further, patients on active surveillance for GG1 disease who are found to have eligible GG2 lesions on surveillance biopsy may be candidates for partial gland water vapor ablation as an alternative to definitive therapy.
This feasibility study while limited in sample size provides data to support further study in a larger clinical trial. Targeted water vapor ablation exhibits potential as a partial gland therapeutic procedure to manage localized PCa and warrants further evaluation.
Abbreviations
AE, adverse event; CTCAE, Common Terminology Criteria for Adverse Events; ED, erectile dysfunction; EjD, ejaculatory dysfunction; EPIC, Expanded Prostate Cancer Index Composite; GG, Gleason Grade Group; IIEF-EF, International Index of Erectile Function-Erectile Function; I-PSS, International Prostate Symptom Score; mpMRI, multiparametric magnetic resonance imaging; MSHQ-EjD-SF, Male Sexual Health Questionnaire-Ejaculatory Dysfunction-Short Form; PCa, prostate cancer; PI-RADS, Prostate Imaging-Reporting and Data System; PSA, prostate specific antigen; QOL, quality of life; TRUS, transrectal ultrasound.
Data Sharing Statement
The data presented in this study is available on request from the corresponding author upon reasonable request.
Acknowledgment
Support for the study was provided by Francis Medical, Inc., Maple Grove, MN, USA.
The authors acknowledge Michael Hoey for his invention of the technology of water vapor therapy and the Francis Medical team for development and application of the platform for treatment of prostate cancer. The authors thank Christopher H. Cantrill, MD, Richard M. Levin, MD, Aaron J. Milbank, MD, Mikhail Regelman, MD, Ronald F. Tutrone Jr, MD, and Michael A. White, MD for their participation in the clinical trial.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all of these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
C.A.W. has no relevant conflicts of interests to disclose. B.D.S. was an independent reader of MRI scans and was paid for this service by Francis Medical. CMD served as a consultant to Francis Medical during execution of this study and currently is Chief Medical Officer at Francis Medical. He is also a consultant for Rezūm product for Boston Scientific Inc.
References
1. Wang A, O’Connor LP, Yerram NK, et al. Focal therapy for prostate cancer: recent advances and future directions. Clin Adv Hematol Oncol. 2020;18(2):116–125.
2. Bates AS, Ayers J, Kostakopoulos N, et al. A systematic review of focal ablative therapy for clinically localised prostate cancer in comparison with standard management options: limitations of the available evidence and recommendations for clinical practice and further research. Eur Urol Oncol. 2021;4:(3):405–423. doi:10.106/j.eio.2020.12.008
3. O’Connor LP, Ramedani S, Daneshvar M, et al. Future perspective of focal therapy for localized prostate cancer. Future perspective of focal therapy for localized prostate cancer. Asian J Urol. 2021;8(4):354–361. doi:10.1016/j.ajur.2021.04.011
4. Rakauskas A, Marra G, Heigegger I, et al. Focal therapy for prostate cancer: complications and their treatment. EAU-YAU prostate cancer (PCa) working party. Front Surg. 2021;8(Jul12):696242. doi:10.3389/fsurg.2021.696242
5. Ehdaie B, Tempany CM, Holland F, et al. MRI-guided focused ultrasound focal therapy for patients with intermediate-risk prostate cancer: a phase 2b, multicentre study. Lancet Oncol. 2022;23(7):910–918. doi:10.1016/S1470-2045(22)00251-0
6. McVary KT, Gange SN, Gittelman MC, et al. Minimally invasive prostate convective water vapor energy (WAVE) ablation: a multicenter, randomized, controlled study for treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2016;195(5):1529–1538. doi:10.1016/j.juro.2015.10.181
7. McVary KT, Gange SN, Gittelman MC, et al. Erectile and ejaculatory function preserved with convective water vapor energy treatment of LUTS secondary to BPH: randomized controlled study. J Sex Med. 2016;13(6):924–933. doi:10.1016/j.jsxm.2016.03.372
8. McVary KT, Gittelman MC, Goldberg KA, et al. Final 5-year outcomes of the multicenter randomized sham-controlled trial of a water vapor thermal therapy for treatment of moderate to severe lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2021;206(3):715–724. doi:10.1097/JU.0000000000001778
9. Dixon CM, Levin RM, Cantrill CH, et al. Transurethral vapor ablation in patients with intermediate risk localized prostate cancer. J Endourol. 2023;37(2):225–232. doi:10.1089/end.2022.0452
10. PI-RADS™ prostate imaging prostate imaging – reporting and data system. 2019. version 2.1; American College of Radiology. 2019. Available from: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/PI-RADS.
11. Common terminology criteria for adverse events (CTCAE) v5.0. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm.
12. Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56(6):899–905. doi:10.1016/S0090-4295(00)00858-X
13. McNeal JE. The zonal anatomy of the prostate. Prostate. 1981;2(1):35–49. doi:10.1002/pros.2990020105
14. Mynderse LA, Hanson D, Robb RA, et al. Rezūm system water vapor treatment for lower urinary tract symptoms/benign prostatic hyperplasia: validation of convective thermal energy transfer and characterization with magnetic resonance imaging and 3-dimensional renderings. Urology. 2015;86(1):122–127. doi:10.1016/j.urology.2015.03.021
15. Weinstock C, Suzman D, Kluetz P, et al. Development of treatments for localized prostate cancer in patients eligible for active surveillance: FDA oncology center of excellence public workshop. J Urol. 2020;203(1):15–119. doi:10.1097/JU.0000000000000532
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



