Back to Journals » Clinical Ophthalmology » Volume 19
Two-Year Post-Market Surveillance of iStent inject® W Combined with Phacoemulsification in Japanese Open-Angle Glaucoma Eyes
Authors Inatani M, Kohama I, Chu A
Received 19 March 2025
Accepted for publication 26 May 2025
Published 12 June 2025 Volume 2025:19 Pages 1863—1876
DOI https://doi.org/10.2147/OPTH.S524956
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Masaru Inatani,1 Ichiro Kohama,2 Alice Chu3
1Department of Ophthalmology, Faculty of Medical Sciences, University of Fukui, Fukui, Japan; 2Department of Clinical Affairs, Glaukos Japan, Tokyo, Japan; 3Department of Market Access and Medical Affairs, Glaukos Singapore, Singapore
Correspondence: Alice Chu, Glaukos Singapore Pte. Ltd., 21st Floor, Centennial Tower, 3 Temasek Avenue, 039190, Singapore, Email [email protected]
Purpose: To evaluate 2-year safety and effectiveness of iStent inject® W implantation with phacoemulsification in adult Japanese open-angle glaucoma (OAG) patients.
Design: Multicenter, prospective, post-market surveillance.
Methods: Eyes were evaluated preoperatively and at Day 1, Week 1, and Months (M) 1, 3, 6, 12, and 24. Primary outcome was M24 cumulative probability of success defined as no additional glaucoma surgery and intraocular pressure (IOP) < preoperative value and number of glaucoma medications ≤ preoperative value or IOP ≤ preoperative value and number of glaucoma medications < preoperative value. Other endpoints included cumulative probability of achieving American Academy of Ophthalmology Glaucoma (AAO) success criteria for minimally invasive glaucoma surgery with phacoemulsification, and changes in mean IOP, number of glaucoma medications, and medication costs over time. Subanalysis was based on OAG subtype [primary open-angle glaucoma (POAG), normal tension glaucoma (NTG), and exfoliative glaucoma (XFG)]. Adverse events were recorded.
Results: Cumulative probability of success at M24 was 91.7%, 98.8%, 88.9%, and 85.0% for cohort (N = 214), NTG, XFG, and POAG, respectively. The AAO M24 success was 80.7%. Statistically significant reductions in mean IOP and number of medications were observed through M24 in cohort and OAG subtypes. At M24, the estimated reductions in mean (standard error) IOP and number of medications were 2.0 (0.5) mmHg and 1.9 (0.4), respectively, in the cohort. Adverse events were minimal. At M24, the average monthly glaucoma medication costs decreased by 49.7%.
Conclusion: Japanese OAG eyes treated with iStent inject W combined with phacoemulsification experienced reduced IOP and medication burden with minimal adverse events and high success rates over postoperative 2 years.
Keywords: minimally invasive glaucoma surgery, trabecular micro-bypass, Japan
Introduction
The prevalence of glaucoma increases with age and is expected to rise with the advancing aging population in Japan, as in other parts of the world.1,2 The Tajimi Study estimated the prevalence of primary open-angle glaucoma (POAG) to be 3.9% in those 40 years and older.1 Most of these cases (92%) had normal tension glaucoma (NTG) with intraocular pressure (IOP) levels of 21 mmHg or less. The more recent Hisayama study approximated a prevalence of 5.8% for POAG.3 Risk factors for POAG in the study were elevated IOP, longer axial length, reduced central corneal thickness, aging, and lower estimated glomerular filtration rate.3
Topical IOP-lowering medications are usually first-line treatment. While effective, they have several drawbacks, including preservative-related ocular surface disease, systemic and local side effects that can reduce patient adherence, and challenges with patient self-management—such as complex dosing regimens and difficulties with self-administration—that can undermine their full therapeutic potential.4,5 Chronic use of these medications can cause deleterious effects on the ocular surface, including dry eye, conjunctival hyperemia, tear film instability, and disruption of corneal epithelial tight junctions.6 Conjunctival inflammation associated with chronic use of topical ocular medications may increase the risk of glaucoma surgery failure.7–10 Side effects, such as irritation from topical medications leading to pain and discomfort, can reduce patient adherence.11,12 Compliance also tends to decrease with the use of multiple medications.13 Ineffective eye drop instillation techniques can lead to medication waste and increased treatment costs.14 Collectively, these factors can contribute to a reduced quality of life for the glaucoma patient.5
Glaucoma surgical innovations have made it more feasible to adopt a proactive, interventional strategy to overcome the challenges associated with topical IOP-lowering medications.4,5,15 Advancements in laser technology such as multipulse selective laser trabeculoplasty [eg, IQ 577 nmTM yellow laser (Iridex, Mountain View, California, United States)],16 the development of sustained-release drug delivery implants [eg, iDose® TR (Glaukos Corporation, Aliso Viejo, California, United States)],17 and the proliferation of minimally invasive glaucoma surgery (MIGS) procedures5 shift the glaucoma treatment paradigm towards earlier surgical intervention. These new therapies offer attributes that address many of the limitations of topical glaucoma medication treatment, supporting the preservation of visual function and improving patients’ quality of life.4,5
In recent years, MIGS has emerged as a safe and effective approach to delay the need for traditional bleb-forming glaucoma surgeries.18 Non-bleb forming implants, such as trabecular micro-bypass stents, coupled with MIGS have expanded the clinicians’ array of tools for treating patients with mild to moderate open-angle glaucoma (OAG). These surgeries are less invasive, resulting in faster recovery, minimal discomfort, fewer postoperative visits, and a lower risk of vision-threatening postoperative adverse events (AE) compared to bleb-forming procedures.15,19 Trabecular micro-bypass stents reduce IOP by restoring the eye’s natural aqueous humor outflow pathway by creating conduits from the anterior chamber into Schlemm’s canal.18 The first generation iStent® (Glaukos Corporation, Aliso Viejo, California, United States), which consisted of 1 titanium, L-shaped stent (1.00 mm by 0.33 mm), was launched in Japan in 2016 for use in combination with phacoemulsification in patients with mild to moderate OAG currently treated with ocular hypotensive medications.20 A study on glaucoma-related procedures from 720 hospitals across Japan revealed a 49% increase in the number of iStent implantation procedures from 2018 to 2019.19 iStent inject® W was launched in Japan in 2020 with the same indication as iStent. This second-generation trabecular micro-bypass system comprises 2 redesigned titanium stents (0.36 mm by 0.36 mm) that provide multi-directional flow in the Schlemm’s canal.21
Both the first generation iStent and its successors, iStent inject and iStent inject W, have demonstrated favorable safety profiles and durability in lowering IOP and reducing the topical medication burden as a standalone procedure or with phacoemulsification.20–25 Greater reductions in IOP have been reported with iStent inject compared with iStent in patients with OAG combined with phacoemulsification in non-Japanese eyes.26,27 However, few studies have evaluated the safety and efficacy of iStent inject W implantation in Japanese patients.24,28 Kanda et al found comparable IOP reductions but greater reductions in mean number of medications in Japanese mild to moderate OAG patients who underwent iStent inject W implantation with phacoemulsification versus those who received iStent,24 while Morita et al28 observed similar clinical outcomes between groups. This 2-year post-market surveillance study was initiated in conjunction with Japan’s Pharmaceuticals and Medical Devices Agency to confirm the safety and effectiveness of iStent inject W, when combined with phacoemulsification, in Japanese OAG patients using pre-specified primary success criteria.
Materials and Methods
The intended study population consisted of 200 adults with mild to moderate OAG [POAG; NTG; exfoliative glaucoma (XFG)] who were receiving ocular hypotensive medications and required phacoemulsification. Participants were to be recruited from 30 sites across Japan. Glaucomatous eyes with an open anterior chamber angle were defined as “primary” if previously recorded IOP was greater than 21 mmHg without an underlying cause; “normal-tension” if previously recorded IOP was 21 mmHg or below without an underlying cause; and “exfoliative” if previously recorded IOP was greater than 21 mmHg with exfoliative material deposits in the trabecular meshwork. iStent inject W implantation was combined with phacoemulsification. This post-market surveillance study was designed to capture real-world outcomes; therefore, no glaucoma medication washout protocol was employed. In addition, surgical procedures and postoperative care, including the adjustment of glaucoma medications, were determined at the discretion of the treating surgeons. The procedures were performed during September 2020 to October 2021. Study time points included the preoperative visit and postoperative assessments at Day 1; Week 1; and Months (M) 1, 3, 6, 12 and 24. This post-market surveillance followed the Japanese Ministerial Ordinance on Good Post-Marketing Study Practice; hence, ethical approval from institutions and patient consent were not required. The study adhered to the principles of the Helsinki Declaration of 1964 and its later amendments.
Study Outcomes
Outcomes were assessed in the cohort and by OAG subtype (POAG, NTG, and XFG). The primary outcome was the cumulative probability of success at M24 defined in Table 1. Success was measured starting at M3. Inability to achieve success on 2 consecutive time points was considered a failure; additional glaucoma surgeries were considered immediate failures.
 |
Table 1 Effectiveness Definitions |
Other outcomes included changes in mean IOP and number of glaucoma medications over time, proportional analysis at M24 vs preoperative time point based on IOP (≤15 mmHg, >15 to 18 mmHg, and >18 mmHg) and number of glaucoma medications (0, 1, 2, or 3 to 5), and incidences of AE. The number of medications was based on medication class, ie, a combination medication would be counted as 2. Mean best-corrected visual acuity (decimal), visual field mean deviation, and corneal endothelial cell density were assessed at M24 and compared with preoperative values.
Because the American Academy of Ophthalmology’s Glaucoma Preferred Practice Pattern® Committee (AAO) developed a success criteria for MIGS with phacoemulsification (ie, no additional laser or incisional glaucoma surgery, loss of light perception vision, or hypotony, and at least 1 glaucoma medication decrease without an increase in IOP, or IOP of 21 mmHg or less and minimum reduction of 20% from preoperative time point without an increase in number of glaucoma medications) after the completion of this study’s data collection, a post hoc analysis was conducted to compare the study’s success against the AAO’s minimal clinically important difference of 65% at M24.15 Uncontrolled IOP or hypotony had to occur over 2 consecutive postoperative visits to be considered a failure. In addition, because medication brands were recorded at each time point, a post hoc analysis was conducted to quantify the economic value of changes in glaucoma medication burden. Pharmaceutical costs were obtained from the Drug Price and Package Insert Search Site.29
Statistical Analysis
Descriptive statistics consisted of mean and standard deviation for continuous variables and frequency and percentages for count variables. Paired t-test was used to compare preoperative and M24 means in eyes with M24 data. A P ≤ 0.05 indicated statistical significance.
The cumulative probabilities of achieving the primary success outcome and meeting the AAO success criteria at M24 were assessed using the Kaplan-Meier method, followed by log-rank testing. Cox proportional hazard regression modeling was used to evaluate OAG subtypes.
Multilevel mixed-effects regression modeling was employed to account for the longitudinal design of the study, with patients nested within surgeons, and surgeons nested within institutions. The predicted changes in mean IOP and glaucoma medication costs over time were calculated using multilevel mixed-effects linear regression. Multilevel mixed-effects Poisson regression was used to estimate the changes in mean number of glaucoma medications over time. If an eye underwent additional glaucoma surgery, its IOP and medication data were censored from all subsequent time points. Multilevel mixed-effects ordered probit regression was used to analyze the changes in ordinal categorical variables (IOP and number of glaucoma medications ranges) at M24 from preoperative time point.
Results
Demographics and Disease Characteristics
The study initially included 216 eyes; however, 2 eyes were excluded because one patient withdrew consent, and the other patient had the generation 1 iStent implanted. Therefore, 214 eyes were analyzed, with procedures performed by 22 surgeons from 14 institutions. Most eyes were either POAG (48.6%) or NTG (46.3%), with 79.6% classified as having mild to moderate disease severity (Table 2). The mean IOP was ≤18 mmHg in 81.8% of eyes. The percentage of eyes on a regimen of 2 or more glaucoma medications was 66.8%. Only 10 eyes had previous glaucoma surgery: 6 laser trabeculoplasties, 4 trabeculotomies, and 1 laser iridotomy.
 |
Table 2 Demographics and Preoperative Characteristics |
 |
Table 3 Observed and Predicted Mean IOP and Number of Glaucoma Medications in Cohort and OAG Subtypes |
During the surveillance period, 43 eyes were considered lost to follow-up due to reasons such as patient death, relocation, or unknown causes.
Success Criteria
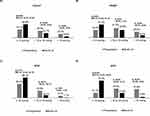
The cumulative success rate (primary outcome) at M24 for the cohort was 91.7% [95% confidence interval (CI): 86.6%, 94.9%] (Figure 1A). The OAG subtypes had cumulative probability of success of 98.8% (95% CI: 91.6%, 99.8%) for NTG, 88.9% (95% CI: 43.3%, 98.4%) for XFG, and 85.0% (95% CI: 75.6%, 91.0%) for POAG (log-rank test: P = 0.004) (Figure 1B). The NTG cohort had a significantly lower hazard of failure compared with the POAG group [hazard ratio (HR): 0.07; 95% CI: 0.01, 0.57; P = 0.012]. No statistically significant difference in the hazard of failure was observed between the XFG and POAG groups (HR: 0.82; 95% CI: 0.11, 6.29; P = 0.851). The wide confidence interval suggests substantial uncertainty in the estimate, likely reflecting the limited sample size in the XFG subgroup.
Based on the AAO success criteria, the cohort’s cumulative probability of success at M24 was 80.7% (95% CI: 74.4%, 85.7%) (Figure 2A). For the OAG subtype analysis using the AAO success criteria,15 the cumulative success rates at M24 were 88.9% (95% CI: 43.3%, 98.4%) for XFG, 88.0% (95% CI: 79.4%, 93.2%) for NTG, and 72.9% (95% CI: 62.5%, 80.8%) for POAG (log-rank test: P = 0.039) (Figure 2B). Cox proportional hazard regression demonstrated a statistically signficant lower hazard of failure in the NTG group compared to the POAG reference group (HR: 0.44, 95% CI: 0.21, 0.88, P = 0.021). No significant difference was observed between XFG and POAG groups (HR: 0.41, 95% CI: 0.06, 3.00, P = 0.377).
Changes in Mean IOP and Number of Glaucoma Medications
Statistically significant changes in mean IOP and number of medications from preoperative values were observed at all postoperative time points in the cohort (Table 3A). In eyes with M24 data (n = 168), the mean IOP and number of medications (standard deviation) were reduced by 2.1 (3.4) mmHg [15.8 mmHg to 13.7 mmHg; P < 0.001] and 1.3 (1.2) [2.3 to 1.0; P < 0.001], respectively. At M24, the predicted reductions in mean (standard error) IOP and number of glaucoma medications were estimated to be 2.0 (0.5) mmHg and 1.9 (0.4) [both P < 0.001], respectively.
All OAG subtypes experienced statistically significant reductions from preoperative time point in both mean IOP and number of medications that were maintained through M24 (Table 3B–D). Among eyes with available M24 data, the POAG group had a mean IOP reduction of 2.0 (4.0) mmHg [from 17.0 mmHg to 15.0 mmHg; P < 0.001], the NTG cohort showed a reduction of 2.0 (2.5) mmHg [from 14.4 mmHg to 12.4 mmHg; P < 0.001], and the XFG cohort had a reduction of 4.7 (3.8) mmHg [from 18.6 mmHg to 13.9 mmHg; P = 0.016]. Mean number of medications was significantly reduced by 1.2 (1.4) [from 2.7 to 1.5; P < 0.001] in POAG, 1.4 (1.1) [from 1.9 to 0.5; P < 0.001] in NTG, and 1.7 (1.5) [from 2.4 to 0.7; P = 0.023] in XFG.
The predicted reductions in mean IOP and number of medications at M24 were 1.8 (0.6) mmHg [P = 0.001] and 1.3 (0.2) [P < 0.001] for POAG, 2.0 (0.6) mmHg [P < 0.001] and 2.6 (1.0) [P = 0.009] for NTG, and 4.5 (1.6) mmHg [P = 0.006] and 2.3 (1.0) [P = 0.020] for XFG, respectively (Table 3B–D).
Proportional Analysis
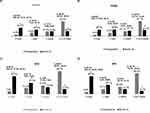
Following iStent inject W implantation with phacoemulsification, a significant shift toward lower IOP was observed in the cohort from the preoperative period to M24 (all P < 0.001) (Figure 3A). The proportion of eyes achieving ≤15 mmHg grew from 43.1% to 69.9% in the cohort, resulting in an absolute percentage point change of 26.8% (95% CI: 15.7%, 37.9%; P < 0.001) (Figure 3A). Similar trends were observed in all 3 OAG subtypes (Figure 3B–D).
iStent inject W implantation resulted in reduced glaucoma medication burden in the overall cohort and across OAG subtypes (Figure 4A–D). At M24, 34.1% of eyes in the cohort were estimated to be medication-free, compared with only 6.5% at preoperative time point — an increase of 27.6% percentage points (95% CI: 12.1%, 43.1%; P < 0.001) (Figure 4A). In addition, all OAG subtypes exhibited a statistically significant increase in the proportion of medication-free eyes at M24 vs preoperative time point (all P < 0.01) (Figure 4B–D).
Among cohort eyes with available data at M24, 37.5% had a reduction of 2 or more glaucoma medications, 42.9% had a reduction of 1 medication, 13.1% had no change, 5.4% required 1 additional medication, and 1.2% required 2 additional medications compared to preoperative values.
Safety
Adverse events, including device-related events, were minimal and manageable. The most common AE was transient IOP increase with 6 events (2.8%) (Table 4). Interventions for this AE included 1 acetazolamide administration, 2 selective laser trabeculoplasties (SLT) and 2 anterior chamber puncture/lavage. For posterior capsule opacification, interventions consisted of 1 YAG laser and 1 secondary cataract incision. One PreserFlo® MicroShunt implantation and 1 trabeculectomy were treatments for progression of visual field defects. One case of corneal edema resolved following anterior chamber lavage, and prescription of oral prednisone for hypopyon. YAG laser was used to treat the single case of peripheral anterior synechiae. In total, only 4 (2%) eyes required additional glaucoma surgery (2 SLTs, 1 PreserFlo MicroShunt, and 1 trabeculectomy) as interventions for the AEs stated above.
 |
Table 4 Adverse Events |
Sixteen device-related AE were reported. The most common was stent occlusion (9 events). Four cases received intervention with YAG laser; the rest either resolved without treatment or were untreated. None of the other device-related AEs required interventions (Table 4).
In eyes with M24 data, best-corrected visual acuity (decimal) improved from mean (standard deviation) of 0.7 (0.3) to 1.0 (0.3) [P < 0.001]. No statistically significant changes were detected in mean visual field mean deviation [preoperative: −7.3 (6.1) dB; M24: −7.4 (6.7) dB; P = 0.920]. Mean corneal endothelial cell density decreased significantly from 2,581 (351) cells/mm2 to 2,487 (324) cells/mm2 (P = 0.002).
Medication Costs
Predicted mean monthly glaucoma medication costs were statistically significantly reduced from preoperative values at all postoperative time points in both the overall cohort and across all OAG subtypes (Table 5). At M24, the estimated percentage reductions in mean monthly glaucoma medication costs compared to the preoperative time point were 49.7% for the overall cohort, 42.6% for POAG, 62.9% for NTG, and 50.3% for XFG. In eyes with available M24 data in the cohort, mean monthly medication costs were reduced by ¥1,637 (128) [from ¥2,665 to ¥1,028; P < 0.001], at M24 vs preoperative time point, indicating a 61.4% reduction. For POAG, NTG, and XFG subgroups, the cost reductions in eyes with M24 data were ¥1,551 (198) [from ¥3,090 to ¥1,540; P < 0.001], ¥1,718 (161) [from ¥2,245 to ¥528; P < 0.001], and ¥1,686 (992) [from ¥2,668 to ¥982; P = 0.140], respectively.
 |
Table 5 Observed and Predicted Mean Monthly Glaucoma Medication Costs in Japanese Yen in Cohort and OAG Subtypes |
Discussion
This 2-year Japan post-market surveillance of iStent inject W combined with phacoemulsification is, to date, the largest study of iStent inject W in Asian eyes. The results demonstrated clinically relevant reductions in both IOP and topical ocular hypotensive medication use compared with preoperative values in OAG eyes. The benefits of medication reductions were translated into medication cost savings that would benefit the government and patients. Furthermore, patients achieved these outcomes with minimal AEs. These findings were also evident in OAG subtypes (POAG, NTG, and XFG).
High success rates were observed overall (91.7%), particularly in NTG eyes (98.8%). Using the AAO success criteria, the cohort’s cumulative success rate at M24 was 80.7%. The AAO successes in OAG subtypes ranged from 73% to 89%. These percentages were higher than the AAO recommended minimal clinically important difference of 65% for the cumulative probability of surgical success for MIGS combined with phacoemulsification at 2 years, indicating benefits to the individual patient.15 Furthermore, the proportion of eyes achieving ≤15 mmHg was estimated to be 69.9% vs 43.1% (preoperative). The improvements in clinical outcomes were accompanied by a favorable safety profile with minimal adverse and device-related events. In addition, significant reductions in medication costs were observed following iStent inject W implantation with phacoemulsification.
The clinical and safety results corroborate those studies in non-Asian, other Asian, and Japanese glaucoma patient populations implanted with iStent inject in combination with phacoemulsification. A 3-year multicentered Australian retrospective study from Clement et al30 in glaucoma eyes (approximately 78% OAG) with 30% having prior glaucoma laser and surgical procedures demonstrated mean IOP reductions from 16.4 mmHg (preoperative) to 13.9 mmHg (P < 0.001). Mean number of glaucoma medications was also reduced from 1.5 to 0.5 (P < 0.001). The proportion of eyes achieving ≤15 mmHg increased from 43.4% (preoperative) to 69.8% (Year 3). Only 4.4% of eyes required additional glaucoma surgery.
A 1-year Singaporean retrospective study on iStent inject combined with phacoemulsification in mainly ethnic Chinese POAG eyes showed a mean IOP reduction of 1.3 mmHg (P = 0.036) from 16.2 mmHg preoperative and reductions in mean number of glaucoma medication of 1.4 (P < 0.001).31 Similar to our study, the achievement of ≤15 mmHg increased from 43.4% to 69.8%. Adverse events were minimal, with only 1 out of 95 eyes requiring rescue glaucoma surgery.
Most published studies from Japan compared iStent inject W to iStent or other MIGS in combination with phacoemulsification.24,28,32,33 Kanda et al investigated the differences in mean IOP and number of glaucoma medications in mild to moderate OAG eyes without prior glaucoma surgery that underwent iStent inject W or iStent implantation with phacoemulsification.24 In the iStent inject W arm, the mean IOP was statistically significantly reduced from 16.5 mmHg to 13.7 mmHg at M12 (P < 0.001). This was similar to our findings in which the observed mean IOP at preoperative at 15.9 mmHg was reduced to 13.2 mmHg at M12. Likewise, their study showed the mean number of medications decreased from 2.3 to 1.0 (P < 0.001), while our observed results indicated a reduction from 2.4 to 0.9. No additional glaucoma surgeries were required in Kanda et al. The only complications reported were 4.4% of eyes with hyphema, which required no anterior chamber washout, and 3.3% of eyes with transient IOP spikes >30 mmHg that were resolved with IOP-lowering medications.
Our study demonstrated favorable outcomes of iStent inject W combined with phacoemulsification in eyes with NTG and XFG. However, as XFG eyes comprised only 5% of the cohort, these results should be considered exploratory and interpreted with caution pending confirmation in larger studies. Few studies on iStent inject technologies have focused exclusively on XFG; however, studies have been published on NTG in combination with phacoemulsification.34,35 A systematic literature review and meta-analysis on angle-based MIGS in NTG from Oo et al, which included studies in Asian eyes, reported a reduction in mean IOP of 2.3 mmHg (95% CI: 1.1, 3.6) and a reduction in mean number of glaucoma medications of 1.1 (95% CI: 0.9, 1.3) at M12 in studies evaluating iStent inject combined with phacoemulsification.36 At M12, our predicted mean IOP reduction was 2.4 mmHg, and the mean reduction in number of glaucoma medications was 2.8 in NTG eyes.
Given the treatment-related burden of glaucoma medications and the growing adoption of MIGS, the glaucoma treatment paradigm is shifting toward a more proactive surgical approach to preserve visual function.4,5,37 The sustained IOP reduction and decreased medication burden associated with MIGS may slow or halt disease progression,38 improve ocular surface health,39,40 and address the challenges of patient self-management4 by reducing reliance on topical medication therapies. Another potential benefit of early surgical treatment is the reduction or elimination of costs associated with long-term use of topical glaucoma medications.5
This single-arm, post-market surveillance was approved by the Japanese authorities to confirm that the safety and clinical outcomes with iStent inject W combined with phacoemulsification previously observed in other populations were also evident in Japanese OAG eyes. The results reflect real-world conditions, as postoperative management and the reintroduction of medications were determined at the discretion of the surgeons. The inclusion of a phacoemulsification-only arm would have provided insight into the additional effectiveness of iStent inject W. The likelihood of regression to the mean was minimized by using repeated measures modeling to account for within-subject variability over time.
Conclusion
In conclusion, iStent inject W implantation combined with phacoemulsification resulted in significantly lower IOP and reduced medication burden from preoperative values, with minimal AE over 24 months of follow-up in Japanese OAG eyes. A 92% success rate at M24 was reported in the overall cohort. These IOP- and medication-lowering benefits were observed across OAG subtypes. Furthermore, the economic value of iStent inject W combined with phacoemulsification was demonstrated through reductions in glaucoma medication costs. Given the high prevalence of NTG in Japan, the observed IOP and medication reductions in this OAG subgroup — along with lower hazard of failure compared to the POAG cohort — provide confidence in the use of this MIGS as a suitable interventional glaucoma treatment option for the Japanese population.
Acknowledgments
The authors would like to thank the principal investigators and their respective institutions for participating in the post-market surveillance. The authors would like to acknowledge Julie Crider, PhD for medical writing contributions. This post-market surveillance was funded by Glaukos Corporation.
Disclosure
Masaru Inatani receives lecture fees and honorarium from Glaukos Japan. Ichiro Kohama is an employee of Glaukos Japan. Alice Chu is an employee of Glaukos Singapore and shareholder of Glaukos Corporation.
References
1. Iwase A, Suzuki Y, Araie M, et al. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology. 2004;111(9):1641–1648. doi:10.1016/j.ophtha.2004.03.029
2. Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi:10.1016/j.ophtha.2014.05.013
3. Fujiwara K, Yasuda M, Hata J, et al. Prevalence of glaucoma and its systemic risk factors in a general Japanese population: the Hisayama Study. Transl Vis Sci Technol. 2022;11(11):11. doi:10.1167/tvst.11.11.11
4. Bedrood S, Berdahl J, Sheybani A, Singh IP. Alternatives to topical glaucoma medication for glaucoma management. Clin Ophthalmol. 2023;17:3899–3913. doi:10.2147/OPTH.S439457
5. Radcliffe NM, Shah M, Samuelson TW. Challenging the “topical medications-first” approach to glaucoma: a treatment paradigm in evolution. Ophthalmol Ther. 2023;12(6):2823–2839. doi:10.1007/s40123-023-00831-9
6. Baudouin C, Labbe A, Liang H, Pauly A, Brignole-Baudouin F. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29(4):312–334. doi:10.1016/j.preteyeres.2010.03.001
7. Broadway D, Hitchings R, Grierson I. Topical antiglaucomatous therapy: adverse effects on the conjunctiva and implications for filtration surgery. J Glaucoma. 1995;4(2):136. doi:10.1097/00061198-199504000-00012
8. Broadway DC, Grierson I, O’Brien C, Hitchings RA. Adverse effects of topical antiglaucoma medication. II. The outcome of filtration surgery. Arch Ophthalmol. 1994;112(11):1446–1454. doi:10.1001/archopht.1994.01090230060021
9. Sherwood MB, Grierson I, Millar L, Hitchings RA. Long-term morphologic effects of antiglaucoma drugs on the conjunctiva and Tenon’s capsule in glaucomatous patients. Ophthalmology. 1989;96(3):327–335. doi:10.1016/s0161-6420(89)32888-0
10. Lavin MJ, Wormald RP, Migdal CS, Hitchings RA. The influence of prior therapy on the success of trabeculectomy. Arch Ophthalmol. 1990;108(11):1543–1548. doi:10.1001/archopht.1990.01070130045027
11. Gatwood J, Brooks C, Meacham R, et al. Facilitators and barriers to glaucoma medication adherence. J Glaucoma. 2022;31(1):31–36. doi:10.1097/IJG.0000000000001965
12. Inoue K, Ishida K, Tomita G, Noma H. A scoping review and network meta-analysis for efficacy and safety of glaucoma medication in Japanese patients. Jpn J Ophthalmol. 2020;64(2):103–113. doi:10.1007/s10384-019-00708-0
13. Kawai-Tsuboi N, Kawai M, Minami Y, Yoshida A. A study of the association between patterns of eye drop prescription and medication usage in glaucoma subjects. J Glaucoma. 2015;24(3):202–206. doi:10.1097/IJG.0b013e31829e1b8b
14. Davis SA, Sleath B, Carpenter DM, Blalock SJ, Muir KW, Budenz DL. Drop instillation and glaucoma. Curr Opin Ophthalmol. 2018;29(2):171–177. doi:10.1097/ICU.0000000000000451
15. Gedde SJ, Vinod K, Bowden EC, et al. Special commentary: reporting clinical endpoints in studies of minimally invasive glaucoma surgery. Ophthalmology. 2024. doi:10.1016/j.ophtha.2024.07.030
16. Zhu D, Shah PP, Zhang C, et al. Outcomes of micropulse laser trabeculoplasty compared to selective laser trabeculoplasty: a systematic review and meta-analysis. Clin Ophthalmol. 2024;18:2205–2215. doi:10.2147/OPTH.S476257
17. Singh IP, Berdahl JP, Sarkisian SR Jr, et al. Long-term safety and efficacy evaluation of travoprost intracameral implant based on pooled analyses from two Phase III trials. Drugs. 2024;84(10):1299–1311. doi:10.1007/s40265-024-02074-9
18. Balas M, Mathew DJ. Minimally invasive glaucoma surgery: a review of the literature. Vision. 2023;7(3):54. doi:10.3390/vision7030054
19. Fujita A, Hashimoto Y, Matsui H, Yasunaga H, Aihara M. Recent trends in glaucoma surgery: a nationwide database study in Japan, 2011-2019. Jpn J Ophthalmol. 2022;66(2):183–192. doi:10.1007/s10384-021-00898-6
20. Inatani M, Kohama I, Chu A. iStent trabecular micro-bypass stent implantation combined with phacoemulsification for open-angle glaucoma: a 2-year post-marketing surveillance study in Japan. Adv Ther. 2022;39(9):4076–4093. doi:10.1007/s12325-022-02207-0
21. Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE, Group USiS. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459–467. doi:10.1016/j.ophtha.2010.07.007
22. Craven ER, Katz LJ, Wells JM, Giamporcaro JE, iStent Study G. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38(8):1339–1345. doi:10.1016/j.jcrs.2012.03.025
23. Hengerer FH, Auffarth GU, Conrad-Hengerer I. 7-year efficacy and safety of iStent inject trabecular micro-bypass in combined and standalone usage. Adv Ther. 2024;41(4):1481–1495. doi:10.1007/s12325-024-02788-y
24. Kanda S, Fujishiro T, Karakawa A, Nakagawa S, Ishii K. Clinical outcomes of phacoemulsification in Japanese patients receiving first and revised second-generation trabecular microbypass stents. Asia Pac J Ophthalmol. 2023;12(3):279–283. doi:10.1097/APO.0000000000000611
25. Lindstrom R, Sarkisian SR, Lewis R, Hovanesian J, Voskanyan L. Four-year outcomes of two second-generation trabecular micro-bypass stents in patients with open-angle glaucoma on one medication. Clin Ophthalmol. 2020;14:71–80. doi:10.2147/OPTH.S235293
26. Paletta Guedes RA, Gravina DM, Paletta Guedes VM, Chaoubah A. Two-year comparative outcomes of first- and second-generation trabecular micro-bypass stents with cataract surgery. Clin Ophthalmol. 2021;15:1861–1873. doi:10.2147/OPTH.S302684
27. Shalaby WS, Lam SS, Arbabi A, et al. iStent versus iStent inject implantation combined with phacoemulsification in open angle glaucoma. Indian J Ophthalmol. 2021;69(9):2488–2495. doi:10.4103/ijo.IJO_308_21
28. Morita S, Sakanishi Y, Riyu I, Watanabe S, Ebihara N. Comparative evaluation of iStent versus iStent inject W combined with phacoemulsification in open angle glaucoma. PLoS One. 2024;19(2):e0297514. doi:10.1371/journal.pone.0297514
29. Drug Price and Package Insert Search Site. Drug price and package insert search site. Available from: https://yakka-search.com/.
30. Clement C, Howes F, Ioannidis A, et al. Multicenter effectiveness and disease stability through 3 years after iStentTrabecular micro-bypass with phacoemulsification in glaucoma and ocular hypertension. Clin Ophthalmol. 2022;16:2955–2968. doi:10.2147/OPTH.S373290
31. Hu Yw J, Ang Ch B, Tecson IOC, Kan Tsia-Chuen J, Yip Wl L. Combined phacoemulsification and iStent inject implantation in Asian eyes. Eur J Ophthalmol. 2022;32(1):288–295. doi:10.1177/11206721211000641
32. Onoe H, Hirooka K, Namiguchi K, et al. Comparison of surgical outcomes between iStent inject W implantation and microhook ab interno trabeculotomy in combination with phacoemulsification in primary open-angle glaucoma patients. Front Med. 2023;10:1266532. doi:10.3389/fmed.2023.1266532
33. Nitta K, Sugiyama K. 2-year success rate of iStent inject W vs. iStent combined with phacoemulsification in reducing intraocular pressure without adjunct glaucoma medications in Japanese open-angle glaucoma eyes. Submitted. 2024.
34. Ang BCH, Chiew W, Yip VCH, et al. Prospective 12-month outcomes of combined iStent inject implantation and phacoemulsification in Asian eyes with normal tension glaucoma. Eye Vis. 2022;9(1):27. doi:10.1186/s40662-022-00294-2
35. Ang BCH, Tecson I, Jyw H, Kan JTC, Yip LWL. 12-month outcomes of combined phacoemulsification and iStent inject in asian eyes with normal tension glaucoma: a single-centre experience. Int Ophthalmol. 2022;42(2):611–620. doi:10.1007/s10792-021-02033-3
36. Oo HH, Hong ASY, Lim SY, Ang BCH. Angle-based minimally invasive glaucoma surgery in normal tension glaucoma: a systematic review and meta-analysis. Clin Exp Ophthalmol. 2024;52(7):740–760. doi:10.1111/ceo.14408
37. Radcliffe N. The case for standalone micro-invasive glaucoma surgery: rethinking the role of surgery in the glaucoma treatment paradigm. Curr Opin Ophthalmol. 2023;34(2):138–145. doi:10.1097/ICU.0000000000000927
38. Gillmann K, Hornbeak DM. Rates of visual field change and functional progression in glaucoma following trabecular microbypass implantation of iStent technologies: a meta-analysis. BMJ Open Ophthalmol. 2024;9(1):e001575. doi:10.1136/bmjophth-2023-001575
39. Samuelson TW, Singh IP, Williamson BK, et al. Quality of life in primary open-angle glaucoma and cataract: an analysis of VFQ-25 and OSDI from the iStent inject(R) pivotal trial. Am J Ophthalmol. 2021;229:220–229. doi:10.1016/j.ajo.2021.03.007
40. Schweitzer JA, Hauser WH, Ibach M, et al. Prospective interventional cohort study of ocular surface disease changes in eyes after trabecular micro-bypass stent(s) implantation (iStent or iStent inject) with Phacoemulsification. Ophthalmol Ther. 2020;9(4):941–953. doi:10.1007/s40123-020-00290-6
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Long-Term Efficacy of Successful Excisional Goniotomy with the Kahook Dual Blade
Wagner IV, Boopathiraj N, Lentz C, Dorairaj EA, Draper C, Kumar D, Checo L, Miller DD, Krambeer C, Dorairaj S
Clinical Ophthalmology 2024, 18:713-721
Published Date: 7 March 2024