Back to Journals » Orthopedic Research and Reviews » Volume 17
Ultrasound-Guided Perineural Corticosteroid Injection for Lacertus Fibrosus Syndrome: A Retrospective Cohort Study
Authors Greene C, Droppelmann G , García N, Jorquera C, Rosales J
Received 22 August 2024
Accepted for publication 2 February 2025
Published 10 June 2025 Volume 2025:17 Pages 229—240
DOI https://doi.org/10.2147/ORR.S492591
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Clark Hung
Cristóbal Greene,1,2 Guillermo Droppelmann,1 Nicolás García,1 Carlos Jorquera,3 Julio Rosales1
1Clínica MEDS, Santiago, Chile; 2Facultad de Medicina, Universidad Diego Portales, Santiago, Chile; 3Facultad de Ciencias, Escuela de Nutrición y Dietética, Universidad Mayor, Santiago, Chile
Correspondence: Guillermo Droppelmann, Clínica MEDS, Santiago, Chile, Email [email protected]
Background: The lacertus fibrosus serves as a site of entrapment for the proximal median nerve. Traditionally, surgical intervention has been the preferred method for resolution. This study demonstrates that perineural corticosteroid injection of the proximal median nerve entrapment under ultrasound guidance can improve nerve compression, strength, and pain in patients with lacertus fibrosus syndrome (LFS).
Methods: A retrospective quasi-experimental cohort study without a control group following the STROBE guidelines was conducted from July 2020 to May 2023. The patient selection was carried out considering Elisabet Hagert’s diagnostic criteria. Ultrasound-guided proximal perineural corticosteroid injections were administered in the region of the lacertus fibrosus. Contingency tables were constructed to compare pre-and post-intervention data. The McNemar test was performed to evaluate the differences. Odds ratios (with 95% CI) were calculated to estimate the likelihood of improvement. A level of less than 0.05 was considered statistically significant. All analyses were performed using the R program.
Results: Twenty-four patients with LFS (61% female, median age: 36 years), were analyzed. Significant improvements were observed in muscle strength perception for the flexor carpi radialis [OR: 33.0, 95% CI: 24.95– 41.0; p < 0.001], index flexor digitorum profundus [OR: 37.0, 95% CI: 29.0– 45.0; p < 0.001], and flexor pollicis longus [OR: 39.0, 95% CI: 31.0– 45.0; p < 0.001]. The scratch test positivity significantly decreased [OR: 4.56, 95% CI: 1.94– 15.67; p < 0.001], and pain levels were notably reduced [OR: 2.33, 95% CI: 0.97– 5.63; p < 0.001].
Conclusion: Perineural corticosteroid injection under ultrasound guidance proved to be a minimally invasive approach for managing LFS. The intervention significantly improves muscle strength perception and reduces nerve compression and pain. These findings underscore the potential of this technique as a viable option for patients who have exhausted other therapeutic approaches before considering surgery.
Level of Evidence: III cohort, treatment study.
Keywords: interventional radiology, lacertus fibrosus syndrome, musculoskeletal ultrasound, ultrasound-guided infiltration
Introduction
Lacertus fibrosus syndrome (LFS) is an uncommon musculoskeletal condition that can cause pain in the elbow and forearm with functional impairment of the muscles innervate by the median nerve. Although its individual prevalence is not well documented, it has been reported to occur in 6% to 13% of cases in conjunction with carpal tunnel syndrome. This is the result of the proximal median nerve entrapment (PMNE) under the ligamentous bundle called Lacertus Fibrosus (LF).1 This expansive aponeurotic sheet emerges from the distal tendon of the biceps brachii, unfolding in a triangular fashion as it extends medially and distally. It traverses the collective muscle mass of the flexors, seamlessly integrating with its fascia.2 LF maintains the rhythmicity between the elbow flexion and supination of the forearm. Specially, increases the lever arm during flexion and supination. It limits the flexion and abduction of the elbow and supination of the forearm.3 It is believed that one of the main causes of LFS is the biomechanical role of this aponeurosis during flexion and the restriction it exerts during elbow supination, which can sometimes result in dynamic nerve compression.4
In LFS, the LF structure serves as a compressor of the pronator teres muscle (PT) during muscle expansion, causing clinical impacts such as pronator syndrome (PS),5 or anterior interosseous nerve entrapment. These conditions can have similar symptoms, making accurate diagnosis difficult in some cases. However, LFS is characterized by its dynamic nature, with symptoms intensifying after physical activity or repetitive efforts.6 In Figure 1, the main anatomical structures around the LF can be observed.
Before the incorporation of imaging technology, diagnosing LFS required measuring compartment pressures, a complex and risky procedure due to the presence of neurovascular bundles.7 Furthermore, LFS is often underdiagnosed, particularly in cases of double crush syndromes involving the median nerve, requiring a thorough analysis of the diagnostic and therapeutic approach to this condition.8 One potential diagnostic strategy is to use ultrasonography (US), which is cost-effective for detecting soft tissue pathologies. However, US has limited sensitivity for detecting LFS, necessitating alternative diagnostic methods.9 Magnetic resonance imaging (MRI) is one such alternative10 and may be included in exercise protocols to improve detection, although it can be cumbersome in practice.6 Although electromyography can be considered the gold standard study for median nerve entrapment in this condition, it is generally negative. For this reason, Hagert has proposed that the diagnosis of the PMNE should be strongly based on a thorough clinical examination.11
In recent years, several US procedures demonstrating favorable clinical and functional outcomes in decompressing the region involving the LF within the PT have been published in scientific literature.12 However, the most notable studies on this subject have predominantly focused on minimally invasive surgical interventions, and the majority of decompressions continue to be performed within a surgical procedure.4 While these approaches show promise, larger studies are required to further investigate their efficacy and potential complications. Additionally, non-invasive treatments such as physical therapy and medication should be considered before opting for surgical intervention.13
An alternative non-surgical option involves perineural infiltration guided by US with corticosteroids. This procedure is typically considered when there is a diagnostic suspicion, and standard medical examinations do not provide a conclusive diagnosis. It is also considered in cases where a clinical diagnosis aligns with the observed signs and symptoms. Moreover, this approach can serve as a therapeutic alternative before undergoing neural release intervention in a surgical setting. To date, no established corticosteroid infiltration procedures for treating LFS have been documented.14,15 A systematic review has recently supported the use of corticosteroids in peripheral nerve pathologies, despite their limited clinical application in the literature. This is because corticosteroids’ anti-inflammatory and regenerative effects can be valuable tools for managing peripheral neuropathies and associated pain.16
For this reason, this study aims to demonstrate that perineural corticosteroid injection in the proximal median nerve entrapment under ultrasound guidance can improve strength muscle perception, nerve compression and pain in patients diagnosed with LFS.
Methods
Ethics Statement
This research adheres to the latest version of the Declaration of Helsinki and the scientific regulations of Chile. To ensure participants’ privacy, stringent measures were implemented, including the anonymization and confidential handling of all collected data. This study was approved by the “Comité de Ética Científico Adulto del Servicio Metropolitano Oriente de la ciudad de Santiago de Chile (SSMO)”, an ethical committee. The project received its approval on May 02, 2023. Given the retrospective design of the study, obtaining informed consent was not required by the ethical committee.
Study Design
A retrospective quasi-experimental cohort study without a control group was performed. A single-site study were conducted. The recommendations of the STROBE Statement guidelines for cohort design were followed.17 All patient records with a diagnosis of LFS who underwent an US-guided perineural corticosteroid injection procedure at the MEDS Clinic in Santiago, Región Metropolitana, Chile were selected.
Patient Selection
Database examination was conducted to determine the number of individuals who underwent the proposed radiological procedure. The study covered 38 months, from March 2020 to May 2023, employing the International Classification of Diseases (ICD-10) systems as advocated by the World Health Organization. Specifically, code G56.10 was utilized, defining other unspecified lesions of the median nerve in the upper limb without age or gender restrictions, excluding carpal tunnel syndrome.18
Patients within the selection criteria were those with PMNE who had undergone US-guided perineural corticosteroid infiltration in the LF region at our institution. Individuals who underwent a surgical release procedure of the LF were excluded from the study.
Clinical Diagnosis
Starting from March 6, 2020, our team of specialized hand surgeons, in collaboration with the interventional radiology team, established a patient database for those with a suspected diagnosis of PMNE due to the LFS. The primary objective was to document the clinical changes in patients who underwent perineural corticosteroid infiltration guided by US.
Diagnosis was established based on the criteria proposed by Elisabet Hagert’s11 including: (i) weakness in muscles innervated by the median nerve distal to the LF such as the flexor carpi radialis (FCR), index flexor digitorum profundus (FDP), and flexor pollicis longus (FPL); (ii) pain or paresthesia upon pressure over the median nerve at the level of the LF; and (iii) positive scratch collapse test (SCT). Electromyographic tests were not utilized for diagnosis. All patients had previously undergone treatment with oral steroids or oral anti-inflammatories, combined with the use of a night splint.
Sample Size
A sample size calculation was performed to detect at least a 30% difference in the proportion before and after the intervention. The McNemar test for paired data was used as the statistical method. A significance level of 0.05 and a power of 80% were set. The required sample size was determined to be 21 patients to ensure the expected effect size.
Following the recommendations for quasi-experimental studies, it may not be ethically required to include a control group in cases where the intervention has demonstrated efficacy and safety,19 such as corticosteroid injections guided by ultrasound. Additionally, this strategy is based on the fact that the patients included chose this option as their last therapeutic alternative before surgery. As noted, participants had already undergone prior treatments, including oral steroids or anti-inflammatories combined with the use of a night splint, without achieving satisfactory results. Consequently, the pre-post-intervention design was considered the most ethical and practical approach to assess the effectiveness of this treatment in improving patient outcomes.
Ultrasound Technique
To ensure optimal conditions, it is recommended to perform the intervention in a dedicated radiological suite equipped for invasive radiological procedures. This room should adhere to strict aseptic measures, include facilities for vital sign monitoring, and meet safety standards. Furthermore, precise localization of the structures surrounding the LF is essential. To achieve this, the procedure is performed by a specialist experienced in US-guided infiltrations for musculoskeletal conditions. For this purpose, an Aplio 500 US system (Toshiba America Medical Systems, Inc., Tustin, CA) with a multifrequency linear transducer is selected, utilizing a frequency of 18 MHz. Prior to initiating the procedure, it is crucial for the professional and their team to verify the availability of all necessary elements. This ensures the successful execution of the intervention while upholding rigorous standards of safety and asepsis. Figure 2 provides a visual representation of all the tools utilized in this process.
 |
Figure 2 Supplies and a sterile tray are set up on the procedural table. |
The patient was placed in the supine position on a flat table, with the forearm lying on the table in a mid-prone position. Then, US was used to locate the LF and median nerve. First, the biceps distal tendon was located in the antecubital fossa at the level of the myotendinous junction. The LF appears as an echogenic linear structure emerging from the myotendinous junction of the biceps muscle, bridging the brachial artery and median nerve, and connecting to the antebrachial fascia that covers the PT. The anatomical site where the median nerve is covered by the crossing of the PT and LF is marked on the skin as the injection site, Figure 3.
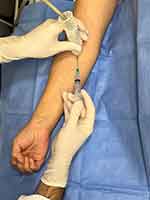
Based on similar experiences in our institution,14,15 the procedure was carried out under strict aseptic conditions; the transducer was inserted into a sterile probe cover after the application of US gel. Infiltration was performed using a 23-gauge needle, with the transducer positioned along the long axis of the median nerve and the needle inserted anatomical plane from distal to proximal, Figure 4. 1 mL of a corticosteroid solution (3 mg of betamethasone acetate and 3.9 mg of betamethasone sodium phosphate; Dacam Rapi-lento®; Laboratorio Chile, Santiago, Chile) was then infiltrated, Figure 5.
A freehand technique was used for injection, with this method, the transducer is held in one hand while the free hand pushes the needle into perineural sites of the median nerve, as perpendicular as possible to the US beam, to identify the route of the needle.
Potential side effects previously reported by some of the authors of this article in corticosteroid infiltration procedures, including local hematoma,20 dizziness, fainting, and low blood pressure, were actively monitored by the professional team during and after the procedure. One-week post-procedure, all patients received a follow-up assessment conducted by the responsible hand surgeon.
Postprocedural Care
Incorporate a partial limitation of activities that involve raising the arm and emphasize the importance of engaging in pain-free activities for the initial 24 hours following the procedure. Additionally, patients are prescribed paracetamol at a dosage of 500 mg every 6 to 8 hours for effective pain management. Furthermore, all patients are informed about the potential occurrence of skin hematoma, an anticipated outcome.
Rehabilitation
The majority of rehabilitation literature addressing median nerve entrapments has concentrated on carpal tunnel syndrome,21,22 showcasing numerous interventions with limited efficacy. Notably, there is a lack of specific protocols for PMNE related to the LF, with reports primarily highlighting immediate strength improvement post-surgical procedures.23 We recommend implementing an accelerated rehabilitation plan three days after the procedure. The focus during the first week should be on reducing pain and inflammation. This should gradually progress to exercises that promote neural mobility of the median, radial, and ulnar nerves using neurodynamic techniques combined with passive and then active exercises. Finally, daily activities, sports, and strength exercises should be introduced to restore full functionality.24
Outcomes Measures
All data was extracted from the specialized team’s database. Key demographic details of the selected patients for this study, such as age, gender, and limb dominance, were taken into consideration. In line with international literature for outcome measurement, the primary variables of interest included the immediate improvement in muscle strength innervated by the median nerve.23 This evaluation encompassed the strength of the FCR during resisted wrist flexion, the strength of the FDP in resisted flexion of the distal interphalangeal joint, and the strength of the FPL in resisted flexion of the thumb’s interphalangeal joint.
The reporting of paresthesias and improvements in pain scores were conducted using an Analog Visual Scale ranging from 0 to 10. Furthermore, the reduction of the SCT sign following the US-guided infiltration procedure was considered. The SCT was measured by the clinician, who resisted bilateral external rotation of the shoulders while keeping the patients’ elbows flexed. After lightly scratching the suspected nerve compression area, the external rotation of the shoulders was immediately repeated with equal resistance. A momentary loss of resistance during external rotation of the affected shoulder is considered a positive sign in the test.25,26 Also, the presence of more than one area of median nerve entrapment was recorded.
Before the radiological procedure, all patients underwent ultrasonographic imaging of the LF, meticulously adhering to specialists’ recommendations to identify all sonographic details in area9. Additionally, the tissue status was assessed in all patients using US post-procedure, and any secondary alteration to the procedure was reported.
All pre-radiological intervention assessments were conducted by an orthopedic specialist who pursued a subspecialty in hand surgery at our institution. This specialist, not among the authors of this article, was chosen to mitigate any potential conflict of interest during the evaluation. Subsequently, post-procedure evaluations were documented by another specialized orthopedic surgeon in hand surgery, who is also unaffiliated with the authors of this project. All assessments were meticulously recorded in the team’s database.
Statistical Analysis
Descriptive statistics were used to analyze the data. Frequencies and medians, along with their respective ranges, were reported. The Shapiro–Wilk test was performed to determine the distribution of the data. Contingency tables were constructed to compare pre- and post-intervention data, categorizing outcomes as improved or unchanged for each variable analyzed. The McNemar test for paired data was then selected to analyze the differences in the variables before and after the procedure.
Additionally, odds ratios (OR) were calculated to estimate the likelihood of improvement after the intervention, with OR values greater than 1 interpreted as indicating a positive effect of the procedure. Confidence intervals (95% CI) for the OR were primarily obtained using bootstrap resampling with 1000 iterations. In cases where the bootstrap confidence intervals were unreasonably narrow or inconsistent, a manual calculation of the 95% CI was performed based on the logarithmic transformation of the OR, using the standard error derived from the contingency table.
A statistical significance level of less than 0.05 was considered using a two-tailed hypothesis. All graphs and analyses were performed using R (The R Foundation for Statistical Computing, v.3.6.2) and RStudio (v.4.1.0).
Results
Participants
Forty patients were selected; however, only twenty-four consecutive patients provided complete information and received both medical and imaging diagnoses of LFS.9
Notably, the majority were female, comprising 61%. The median age was 36 years (ranging from 18 to 64 years). Right-hand dominance was observed in all subjects. Furthermore, 67% of individuals experienced entrapment on the same side of their body, and 20% of the subjects exhibited more than one area of median nerve entrapment. Figure 6 shows the flowchart of the study participants.
 |
Figure 6 Flowchart of the selected participants. |
Outcomes results
The intervention demonstrated a significant impact on clinical and functional outcomes—specifically, muscle strength, neurological examination results, and pain intensity. Regarding muscle strength, assessments of the FCR, FDP, and FPL muscles revealed notable improvements, as reported by the patients. As a result, the probability of recovery increased substantially in each evaluated muscle group (p < 0.001).
Additionally, all patients initially tested positive for the SCT prior to the US-guided procedure. However, following the intervention, only four patients (16%) remained positive, indicating a statistically significant change (p < 0.001). Furthermore, patients were approximately five times more likely to improve after the proposed intervention, demonstrating a strong association between the intervention and the resolution of the clinical sign measured by the SCT.
With respect to pain reduction, outcomes also improved markedly after the US procedure. Although all participants reported pain beforehand, only seven patients (29%) continued to report pain afterward, representing a statistically significant improvement (p < 0.001).
Table 1 summarizes the main OR with their corresponding 95% CI. In addition, it highlights the p-values obtained from the individualized McNemar test for each analyzed variable.
 |
Table 1 Association Analysis (OR, 95% CI, and p-value) for the Evaluated Clinical Variables |
Discussion
The primary objective of this article was to introduce a novel, minimally invasive radiological technique for managing patients with LFS. This approach utilizes a perineural corticosteroid injection under US guidance to target proximal median nerve entrapment, aiming to improve muscle strength, nerve compression, and reduce pain in patients diagnosed with LFS.
While an infrequent diagnosis in the orthopaedic domain, and with a dearth of international scientific literature on minimally invasive procedures, this quasi-experimental cohort of patients, characterized by the application of guided US for the perineural administration of corticosteroids to the proximal median nerve, assumes a notable position. Consequently, perineural corticosteroid infiltration guided by US emerges as a therapeutic modality meriting consideration before contemplating more invasive or surgical interventions.
Traditionally, the surgical option for this musculoskeletal condition has encompassed the liberation of the LF and, occasionally, carpal tunnel release. A study provided evidence through an extensive series of cases that surgical intervention should be the method of choice due to the excellent results that are presented.4 Paradoxically, the article also spotlights certain instances of less invasive experiences. Additionally, another publication described a minimally invasive procedure using the WALANT technique, facilitated by percutaneous US guidance, to release the brachial biceps LF in scenarios of median nerve entrapment at the elbow. The authors emphasized the significant reduction in pain and contingent improvements,12 similar to the report in this article. However, their study has a more limited sample size and lacks statistical analysis.
It is pertinent to note that most scientific articles focus on the distal region of the median nerve, with one addressing the proximal region, favoring techniques that are less invasive and cost-effective. In 2019, Fried et al27 introduced an innovative method using an US-guided external hydrodissection injection to release the median nerve from the carpal tunnel. This injection allowed for a smoother dissection of surrounding tissues compared to open external neurolysis, minimizing the risk of recurrent scarring.
In this context, our proposal aligns with the current international trend, following our established approach of offering interventions that are minimally invasive and cost-effective, once again employing corticosteroids as a therapeutic agent.14 Moreover, we suggest this technique can be utilized in preliminary stages, enhancing US’s diagnostic accuracy, particularly given the region’s complex anatomy and variability.9,28 The technique being studied provides numerous advantages, inclusive of its economic prudence, dual functionality as both a diagnostic and therapeutic instrument, instantaneous alleviation of symptomatic presentation, absence of ionizing radiation, outpatient feasibility, negation of anesthesia and pharmacological administration, alongside its precision.29,30 Nevertheless, we must also accord heed to potential limitations, including the advent of localized hematoma post-procedure, reliance upon adept practitioners, requisite infrastructural prerequisites for interventional radiological endeavors, and plausible interactions of corticosteroids among diabetics with suboptimal glycemic control.31
We emphasize the positive outcomes observed in muscle strength perception, nerve entrapment, and pain, underscoring their clinical relevance and the impact on patients’ quality of life. Nonetheless, our study has several methodological limitations that should be addressed in future research. First, this study is based on a retrospective cohort of consecutive patients, for whom the proposed intervention represented the final therapeutic alternative before surgery. Due to ethical considerations, we employed a quasi-experimental design, which lacks a control group, limiting our ability to establish the procedure’s efficacy fully. Second, the sample size appears small when compared to traditional studies. While the intervention demonstrated a positive impact, as evidenced by the odds ratio and statistical significance, the primary concern should be its external validity. A larger sample size would enable the inclusion of participants whose characteristics better reflect the general population, thereby improving the generalizability of the findings. Additionally, future research should incorporate prospective follow-up to evaluate the long-term effectiveness of the intervention. This would include monitoring symptom recurrence, the need for additional interventions, and exploring the potential benefits of combining treatments, such as a complementary plan of neural mobilizations,32 which may further enhance outcomes for individuals with LFS. Furthermore, long-term studies are needed to fully assess the efficacy and long-term safety of this innovative technique.
Finally, we emphasize multiple advantages. Firstly, being an US-guided procedure, it provides exceptional precision by allowing real-time visualization of the involved anatomical structures. This reduces the risk of unintended injuries and enhances treatment effectiveness. Additionally, being a less invasive approach compared to traditional techniques, it entails a lower likelihood of complications and shorter recovery times for patients. It’s important to consider that this technique may require a certain level of proficiency in US imaging by the operator, which could potentially limit its widespread adoption, as shown in Tables 2 and 3
 |
Table 2 Advantages and Disadvantages |
 |
Table 3 Pearls and Pitfalls |
Conclusions
The proposed intervention significantly improved hand muscle strength perception, neural entrapment signs, and pain in patients with LFS, without secondary complications. Ultrasound is emerging as a minimally invasive, versatile diagnostic and therapeutic tool in the musculoskeletal field. Its simplicity, ease of use by medical staff, and portability position it as a valuable resource with potential for widespread adoption. However, further research is needed, including studies with control groups, larger sample sizes to enhance external validity, prospective follow-ups to assess the duration of effects and potential side effects, and complementary therapies to strengthen outcomes.
In summary, radiological interventions in the musculoskeletal domain can revolutionize clinical practice by enabling real-time decision-making and integrating diagnosis with treatment. This approach offers a safe and viable option for patients who have exhausted other therapeutic alternatives before surgery.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lalonde D. Lacertus syndrome: a commonly missed and misdiagnosed median nerve entrapment syndrome. BMC Proc. 2015;9(3):74. doi:10.1186/1753-6561-9-S3-A74
2. Caetano EB, Vieira LA, Almeida TA, Gonzales LAM, de Bona JE, Simonatto TM. Bicipital aponeurosis. Anatomical study and clinical implications. Rev Bras Ortop. 2017;53(1):75–81. doi:10.1016/J.RBOE.2017.11.014
3. Snoeck O, Coupier J, Beyer B, et al. The biomechanical role of the lacertus fibrosus of the biceps brachii muscle. Surg Radiol Anat. 2021;43(10):1587–1594. doi:10.1007/s00276-021-02739-0
4. Hagert E, Jedeskog U, Hagert CG, Marín Fermín T. Lacertus syndrome: a ten year analysis of two hundred and seventy five minimally invasive surgical decompressions of median nerve entrapment at the elbow. Int Orthop. 2023;47(4):1005. doi:10.1007/S00264-023-05709-W
5. Swiggett R, Ruby LK. Median nerve compression neuropathy by the lacertus fibrosus: report of three cases. J Hand Surg Am. 1986;11(5):700–703. doi:10.1016/S0363-5023(86)80015-6
6. Mehl A, Stevenson J, Royal JT, Lourie GM. Lacertus syndrome: use of pre- and post-exercise MRI to aid in diagnosis and treatment. Radiol Case Reports. 2021;16(5):1113–1117. doi:10.1016/j.radcr.2021.02.022
7. Mark P, Elizabeth O. Acute compartment syndrome of the upper extremity: JAAOS - journal of the American Academy of orthopaedic surgeons. Am Acad Orthop Surg. 2011;16(1):49–58.
8. Abdalbary SA, Abdel-Wahed M, Amr S, et al. The myth of median nerve in forearm and its role in double crush syndrome: a cadaveric study. Front Surg. 2021;8(September):1–8. doi:10.3389/fsurg.2021.648779
9. Konschake M, Stofferin H, Moriggl B. Ultrasound visualization of an underestimated structure: the bicipital aponeurosis. Surg Radiol Anat. 2017;39(12):1317. doi:10.1007/S00276-017-1885-0
10. Lombardi A, Ashir A, Gorbachova T, Taljanovic MS, Chang EY. Magnetic resonance imaging of the elbow. Pol J Radiol. 2020;85(1):e440–e460. doi:10.5114/pjr.2020.98691
11. Hagert E. Clinical diagnosis and wide-awake surgical treatment of proximal median nerve entrapment at the elbow: a prospective study. Hand. 2013;8(1):41. doi:10.1007/S11552-012-9483-4
12. Apard T, Mares O, Duparc F, Michelin P. Percutaneous ultrasound-guided release of the lacertus fibrosus for median nerve entrapment at the elbow. Cardiovasc Intervent Radiol. 2022;45(8):1198–1202. doi:10.1007/S00270-022-03123-0/METRICS
13. Löppönen P, Hulkkonen S, Ryhänen J. Proximal median nerve compression in the differential diagnosis of carpal tunnel syndrome. J Clin Med. 2022;11(14):3988. doi:10.3390/jcm11143988
14. García N, Rosales J, Greene C, Droppelmann G, Verdugo MA. Ultrasound-guided hydraulic release associated with corticosteroids in radial tunnel syndrome: description of technique and preliminary clinical results. J Ultrasound Med. 2020;39(1):165–168. doi:10.1002/JUM.15085
15. Rosales J, García N, Rafols C, Pérez M, Verdugo MA. Perisciatic ultrasound-guided infiltration for treatment of deep gluteal syndrome. J Ultrasound Med. 2015;34(11):2093–2097. doi:10.7863/ULTRA.14.12030
16. Couch B, Hayward D, Baum G, et al. A systematic review of steroid use in peripheral nerve pathologies and treatment. Front Neurol. 2024;15(September):1–13. doi:10.3389/fneur.2024.1434429
17. Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. 2017;89:218–235. doi:10.1016/j.jclinepi.2017.04.026
18. World Health Organization. ICD-10 Version:2019. ICD-10. 2019. Available from: https://icd.who.int/browse10/2019/en.
19. Harris AD, McGregor JC, Perencevich EN, et al. The Use and Interpretation of Quasi-experimental studies in medical informatics. J Am Med Inform Assoc. 2006;13(1):16. doi:10.1197/JAMIA.M1749
20. García N, Droppelmann G, Oliver N, Jorquera C, Rosales J. Nonsurgical management of shoulder pain in rotator cuff tears: ultrasound-guided biceps tenotomy combined with corticosteroid injection. Arthrosc Tech. 2024;13(2):102847. doi:10.1016/J.EATS.2023.09.022
21. Peters S, Page MJ, Coppieters MW, Ross M, Johnston V. Rehabilitation following carpal tunnel release. Cochrane Database Syst Rev. 2016;2016(2). doi:10.1002/14651858.CD004158.PUB3/MEDIA/CDSR/CD004158/IMAGE_N/NCD004158-CMP-004-02.PNG
22. Wielemborek PT, Kapica-Topczewska K, Pogorzelski R, Bartoszuk A, Kochanowicz J, Kułakowska A. Carpal tunnel syndrome conservative treatment: a literature review. Adv Psychiatry Neurol Psychiatr I Neurol. 2022;31(2):85–94. doi:10.5114/PPN.2022.116880
23. Azócar C, Corvalán G, Orellana P, Cobb P, Liendo R, Román J. Intraoperative immediate strength recovery following lacertus fibrosus release in patients with proximal median nerve compression at the elbow. Int Orthop. 2023;47(11):2781–2786. doi:10.1007/S00264-023-05888-6/METRICS
24. Sierra-Silvestre E, Tachrount M, Themistocleous AC, Stewart M, Baskozos G, Schmid AB. Mechanisms of neurodynamic treatments (MONET): a protocol for a mechanistic, randomised, single-blind controlled trial in patients with carpal tunnel syndrome. BMC Musculoskelet Disord. 2024;25(1):1–12. doi:10.1186/S12891-024-07713-6/TABLES/2
25. Cheng CJ, Mackinnon-Patterson B, Beck JL, Mackinnon SE. Scratch collapse test for evaluation of carpal and cubital tunnel syndrome. J Hand Surg Am. 2008;33(9):1518–1524. doi:10.1016/j.jhsa.2008.05.022
26. Jain NS, Zukotynski B, Barr ML, Cortez A, Benhaim P. The scratch-collapse test: a systematic review and statistical analysis. Hand. 2023;1–8. doi:10.1177/15589447231174483
27. Fried SM, Nazarian LN. Ultrasound-guided hydroneurolysis of the median nerve for recurrent carpal tunnel syndrome. Hand. 2019;14(3):413–421. doi:10.1177/1558944717731855
28. Nulle K, Jaudzema A. Ultrasonographic evaluation of the median nerve: normal and variant anatomy and appearance. J Ultrason. 2021;21(87):e318. doi:10.15557/JOU.2021.0053
29. Acebes C, Rubio L, Román A, Herrero A, Arcos J. Cost-effectiveness of on-site musculoskeletal ultrasound in an outpatient rheumatology clinic. Rheumatology. 2021;60(4):1832–1838. doi:10.1093/RHEUMATOLOGY/KEAA678
30. Baloch N, Hasan OH, Jessar MM, Hattori S, Yamada S. “Sports Ultrasound”, advantages, indications and limitations in upper and lower limbs musculoskeletal disorders. Review article. Int J Surg. 2018;54:333–340. doi:10.1016/J.IJSU.2017.11.034
31. Safran O, Fraind-Maya G, Kandel L, Leibowitz G, Beyth S. The effect of steroid injection into the shoulder on glycemia in patients with type 2 diabetes. JSES Int. 2022;6(5):843. doi:10.1016/J.JSEINT.2022.05.016
32. Tedeschi R, Platano D, Melotto G, et al. Effectiveness of neurodynamic treatment in managing lateral epicondylitis: a systematic review. Review article. Manuelle Medizin. 2024;62(4):276–283. doi:10.1007/s00337-024-01063-z
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.