Back to Journals » Orthopedic Research and Reviews » Volume 16
Ultrasound-Guided Suprainguinal Fascia Iliaca Compartment Block in Combination with Sciatic Nerve Block for Pain Reduction in Total Hip Arthroplasty: A Prospective Randomized Controlled Study
Authors Zhou J , Guo M, Wang J , Hu Q , Liu Y, Chen Z , Lu F, Lin Y, Zhong M, Wang L
Received 2 August 2024
Accepted for publication 25 November 2024
Published 27 November 2024 Volume 2024:16 Pages 283—293
DOI https://doi.org/10.2147/ORR.S489775
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Clark Hung
Jianshun Zhou,1,2,* Mingling Guo,3– 5,* Jiasheng Wang,1,4,5 Qian Hu,1,4,5 Yingying Liu,1,4,5 Zhen Chen,1,4,5 Feng Lu,3– 5 Yong Lin,3– 5 Maolin Zhong,3– 5 Lifeng Wang3– 5
1The First Clinical Medical College of Gannan Medical University, Ganzhou, Jiangxi, People’s Republic of China; 2Department of Anesthesiology, Ganzhou Cancer Hospital, Ganzhou, Jiangxi, People’s Republic of China; 3Department of Anesthesiology, First Affiliated Hospital of Gannan Medical University, Ganzhou, Jiangxi, People’s Republic of China; 4Ganzhou Key Laboratory of Anesthesiology, The First Affiliated Hospital of Gannan Medical University, Ganzhou, Jiangxi, People’s Republic of China; 5Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases, Ministry of Education, Gannan Medical University, Ganzhou, Jiangxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lifeng Wang; Maolin Zhong, Email [email protected]; [email protected]
Purpose: The aim of this study was to observe the intraoperative and postoperative analgesic effects of suprainguinal fascia iliaca compartment block (SFICB) combined with sciatic nerve block (SNB) in patients undergoing total hip arthroplasty (THA).
Patients and Methods: Eighty-seven THA patients were randomly assigned to three groups: general anesthesia (Group C), general anesthesia with SFICB (Group F), and general anesthesia with SFICB and SNB (Group F+S). Numeric Rating Scale (NRS) scores were used to evaluate pain levels at rest and during activity at various postoperative time points. The secondary outcomes included heart rate (HR), mean arterial pressure (MAP), intraoperative sufentanil consumption, number of effective presses on the analgesic pump, rescue analgesic administration, postoperative nausea and vomiting, and serum levels of IL-1β and TNF-α.
Results: NRS scores were significantly lower in Groups F and F+S compared to Group C at different postoperative time points both at rest and during activity (P< 0.05). Intraoperative sufentanil consumption, the number of effective presses on the analgesic pump, rescue analgesic administration, and postoperative nausea and vomiting were lower in Groups F and F+S compared to Group C (P< 0.05). There were also significant differences in sufentanil consumption and the number of effective presses on the analgesic pump between groups F and F+S (P< 0.05). The expression levels of IL-1β and TNF-α were lower in groups F and F+S compared to group C (P< 0.05) at specific time points.
Conclusion: The application of ultrasound-guided SFICB combined with SNB for total hip arthroplasty can provide more comprehensive analgesia, reduce postoperative NRS scores, alleviate haemodynamic fluctuations, decrease opioid drug use, and reduce the serum levels of inflammatory factors, especially when combined with SNB.
Keywords: suprainguinal fascia iliaca block, sciatic nerve block, total Hip arthroplasty
Introduction
With the accelerated progression of global ageing, the incidence of hip fractures in elderly individuals is increasing. Early implementation of total hip replacement (THA) is a common method for treating hip fractures.1 THA surgery can cause substantial trauma, excessive bleeding, and complications.2–4 Intraoperative and postoperative pain can induce strong stress responses in the body, leading to the release of many inflammatory factors. These factors may cause central sensitization and lower pain thresholds, exacerbating postoperative pain.5 Postoperative pain can hinder early mobilization, increase the duration of bed rest, and increase the incidence of deep venous thrombosis, atelectasis pneumonia, pressure ulcers, and even chronic pain.6 Therefore, reducing intraoperative pain is particularly important for perioperative safety and rapid postoperative recovery. However, patients who undergo THA are often elderly individuals with multiple underlying medical conditions. Organ decline and decreased medication tolerance increase the perioperative risks associated with simple analgesics.7 Multimodal analgesia is an effective strategy for reducing adverse reactions caused by single drugs or methods.8,9 Nerve blockade, a common form of multimodal analgesia, provides excellent intraoperative and postoperative analgesic effects by reducing the expression levels of inflammatory factors and decreasing the occurrence of postoperative complications.10–12 Numerous studies have shown that fascia iliaca block (FICB) applied to patients with hip fractures can reduce patient demand for opioid drugs during the perioperative period and alleviate postoperative pain.13–15 However, compared with infrainguinal FICB, suprainguinal FICB (SFICB) has been shown to increase the success rate of blocking the lateral cutaneous nerve in the thigh, which provides better postoperative analgesics for hip arthroplasty surgeries.16 Currently, there are few reports on the use of SFICB combined with SNB for analgesia in THA patients. This study aimed to observe the perioperative analgesic effect of ultrasound-guided SFICB combined with SNB for THA to provide a reference for its application in such surgeries.
Materials and Methods
Study Design
This single-centre, prospective, double-blind, and randomized controlled trial was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Gannan Medical University (project number: LLSC-2021082401) and registered in the Chinese Clinical Trial Registry (ChiCTR2100050375||http://www.chictr.org.cn/, date of registration: 26/08/2021). All procedures performed in the study were in accordance with the ethical standards of the Declaration of Helsinki (1964) and its later amendments or comparable ethical standards. All enrolled patients signed informed consent forms.
Study Population
Before randomization, all participants were informed about the potential benefits and risks of this study as well as alternative options and signed informed consent forms.
Inclusion Criteria
(1) aged 60–75 years, undergoing total hip arthroplasty; (2) American Society of Anesthesiologists (ASA) classification I–III; (3) body mass index (BMI) of 18 kg/m2–24 kg/m2; and (4) provided informed consent.
Exclusion Criteria
(1) coagulation disorders; (2) infection at the puncture site; (3) communication difficulties or mental disorders that hinder cooperation; (4) allergy to the local anaesthetics used in this trial; (5) long-term use of analgesic drugs; (6) peripheral nervous system diseases involving the lower limbs; and (7) refusal to participate in the trial.
Patients were excluded after randomization and before analysis in the following cases: (1) surgery time >3 h; (2) failed nerve blockade or local anaesthetic toxicity; (3) request to withdraw from the trial; (4) incomplete postoperative data collection; and (5) the occurrence of adverse drug reactions or wound infections or the need for reoperation for other reasons after the surgery.
Sample Size Calculation
The primary outcome measure of this trial was the postoperative Numeric Rating Scale (NRS) score. Based on the pilot study results, the sample size was calculated multiple times using the mean and standard deviation of the difference between the postoperative NRS score and the preoperative NRS score at different time points in the three groups of THA patients. The maximum sample size was selected to ensure that the differences in NRS scores at each time point were statistically significant. The mean differences in NRS scores (at rest) between the SFICB combined with SNB group, SFICB group, and control group were 3.00, 3.67, and 4.00, respectively, with standard deviations of 0.82, 0.94, and 0.94, respectively. Using a two-tailed test (α=0.05 and β=0.10) and PASS 15.0 software, the sample size for each group was calculated to be 23 patients. Considering a dropout rate of 20%, each group required 29 patients, for a total of 87 patients.
Randomization and Blinding
The random number generator in SPSS 25.0 software was used for randomization. A fixed random seed of 20210801 was set, and random numbers were assigned to each subject via a uniform random function. The subjects were subsequently divided into 3 groups in the order of the generated random numbers, with equal sample sizes. The grouping information was written down and placed in an opaque envelope, and a code was written on the outside of the envelope, which was sealed and handed to the researchers. After the subjects entered the study, they were individually numbered, and the envelope with the corresponding number was opened to assign the subjects to their respective groups according to the allocation information inside the envelope. Patients knew their assigned numbers but not the group allocation. The randomization process was conducted by a research administrator who was not involved in the trial. The nerve blockade procedures were performed by the same anesthesiologist who was not involved in the study, based on the envelope group allocation information. The surgeries were performed by the same primary surgeon. Patients, responsible anaesthesiologists, other members of the medical team, and the researchers responsible for patient recruitment, data collection, and follow-up assessments were unaware of the group assignments. To ensure patient safety, in the event of an emergency situation (such as a severe adverse event) that poses a serious threat to the subject’s safety and when it is necessary to know the subject’s allocation, authorized designated personnel will unblind individual subjects by following the procedures specified in the protocol, as outlined in the emergency unblinding process, for the purpose of case exclusion.
Anaesthesia Method
Upon entering the preoperative anaesthesia room, the patients underwent routine monitoring of blood pressure (BP), oxygen saturation (SpO2), heart rate (HR), and cardiac activity via electrocardiography (ECG). Venous access was established, and radial artery cannulation was performed under local anaesthesia. All three groups of patients in the operating room were administered sufentanil (Yichang Renfu Pharmaceutical Co., Ltd) at a dose of 0.15 μg/kg and midazolam (Jiangsu Enhua Pharmaceutical Co., Ltd) at a dose of 0.03 mg/kg.
Group F underwent ultrasound-guided SFICB. The patient was placed in the supine position, and after routine sterilization and draping, a 5–10 MHz high-frequency linear array ultrasound probe was positioned at the anterior superior iliac spine on the affected side, with the other end directed towards the patient’s navel. The probe was slightly moved to find the clearest ultrasound image and the optimal needle entry position. Ultrasound revealed that the anterior superior iliac spine, iliac muscle, iliopsoas fascial space, transversus abdominis, and internal oblique muscles were visible (Figure 1a). The deep circumflex iliac artery was identified, and a 22G nerve block needle was inserted into the iliopsoas fascial space. After negative blood aspiration, 30 mL of 0.25% ropivacaine was injected in multiple increments. Under ultrasound guidance, the local anaesthetic was injected to separate the iliopsoas fascia from the iliac muscle (Figure 1b).
Patients in Group F+S underwent ultrasound-guided SFICB combined with SNB. SFICB was performed first via the aforementioned method. The sciatic nerve block was performed via a subgluteal approach. The patient was positioned in a lateral decubitus position with the affected limb on top and the hip and knee flexed. The convex array ultrasound probe was placed horizontally over the greater trochanter of the femur and ischial tuberosity, with the gluteus maximus, greater trochanter, ischial tuberosity, and sciatic nerve visible on the ultrasound image (Figure 1c). When the needle tip was close to the target position, the assistant was asked to aspirate the fluid, and if no blood was observed, 20 mL of 0.375% ropivacaine was injected.
Blockade Effect Assessment
Thirty minutes after the nerve blockade procedure was completed, the blockade effect was assessed by an anaesthesiologist who was unaware of the group assignments. Skin punctures were made on the anterior and lateral aspects of the thigh, as well as on the posterior aspect of the thigh, to assess sensory blockade. If the skin in the blocked area was numb, successful blockade was indicated; otherwise, the patient was excluded.
Anaesthesia Management
Anaesthesia Induction
Anaesthesia was induced via sequential intravenous injection of sufentanil (batch number: H20054171, Yichang Renfu Pharmaceutical Co., Ltd., 1 mL: 50 μg/kg) at a dose of 0.3 μg/kg, etomidate (batch number: H20020511, Jiangsu Enhua Pharmaceutical Co., Ltd., 10 mL: 20 μg) at a dose of 0.3 mg/kg, and rocuronium bromide (batch number: H20093186, Zhejiang Xianju Pharmaceutical Co., Ltd., 5 mL: 50 mg) at a dose of 0.6 mg/kg. After endotracheal intubation, mechanical ventilation was initiated, with a respiratory rate of 10–16 breaths/minute and a tidal volume of 6–8 mL/kg to maintain end-tidal carbon dioxide (PetCO2) levels between 35–45 mmHg.
Anaesthesia Maintenance
Propofol was infused at a rate of 4.0–8.0 mg·kg−1·h−1 via an intravenous pump, with intermittent supplementation of rocuronium bromide (5–10 mg) and sufentanil (5–10 μg). The bispectral index (BIS) was maintained between 40 and 60. In the event of bradycardia (HR<50 beats/minute), 0.5 mg of atropine was administered intravenously; for hypotension (mean arterial pressure (MAP)<55 mmHg), 6 mg of ephedrine was administered intravenously. The intravenous infusion of anaesthetic drugs was stopped at the end of the surgery.
Postoperative Analgesia
Postoperative analgesia was provided via patient-controlled intravenous analgesia. The analgesic pump solution consisted of 100 μg sufentanil + 16 mg ondansetron + 100 mL normal saline. The background infusion rate was set at 2 mL/h, with a bolus dose of 2 mL and a lockout time of 15 minutes. If the NRS score at rest was greater than 4, rescue analgesia was administered intravenously with 50 mg tramadol.
Outcome Variables
Primary Outcomes
NRS scores at rest and during movement at 6 h, 12 h, 24 h, and 48 h after surgery for all three groups of patients (0: no pain, 1–3: mild pain, 4–6: moderate pain, 7–10: severe pain) were determined.
Secondary Outcomes
HR and MAP were evaluated at admission (T0), 10 minutes after the completion of anaesthesia (T1), 5 minutes after the start of surgery (T2), and at the end of surgery (T4) for all three groups of patients. The amount of sufentanil used during surgery, the number of effective presses on the analgesic pump within 24 hours after surgery, the number of patients requiring rescue analgesia after surgery, and the incidence of local anaesthetic toxicity and postoperative nausea and vomiting were evaluated for all three groups of patients. Finally, the levels of IL-1β and TNF-α in serum at admission (T0), 30 minutes after the start of surgery (T3), and at the end of surgery (T4) for all three groups of patients were measured.
Serological Indicator Determination
Peripheral venous blood samples of 4 mL were collected from the patients at admission, 30 minutes after the start of surgery, and at the end of surgery. The samples were immediately transferred into nonanticoagulant tubes and centrifuged at 3000 × g for 5 minutes to obtain the serum. The serum was stored at −80°C for subsequent testing. Serum IL-1β and TNF-α concentrations were measured via enzyme-linked immunosorbent assay (ELISA). ELISA kits for IL-1β and TNF-α from Andy Gene Biotechnology Co., Ltd. were used according to the manufacturer’s instructions, and the optical density (OD) values were read at 450 nm via an ELISA reader (Thermo Fisher). A standard curve was plotted with the concentrations of the standard samples on the x-axis and the OD values on the y-axis. The corresponding IL-1β and TNF-α concentrations in each serum sample were calculated by comparing their OD values to those of known standards.
Statistical Analysis
The data collected in the study were processed and analysed via SPSS 25.0 statistical software. Normally distributed continuous data are expressed as the mean ± standard deviation, and one-way analysis of variance was used for intergroup comparisons, followed by Bonferroni correction for pairwise comparisons. Nonnormally distributed continuous data are expressed as the median (M) and interquartile range (IQR), and the Kruskal‒Wallis H-test was used for intergroup comparisons. Categorical data are presented as the number of patients, and Pearson’s chi-square test or Fisher’s exact test was used for intergroup comparisons, followed by chi-square splitting or Fisher’s exact probability test for pairwise comparisons. Bonferroni correction was applied to P values during pairwise comparisons. A significance level of P < 0.05 was considered statistically significant.
Results
A total of 113 patients were assessed for eligibility, with 26 patients excluded (22 patients did not meet the inclusion criteria, and 4 patients declined to participate in the study). A total of 87 patients ultimately participated in the study. One patient each from Groups C and F was excluded from the final statistical analysis because the surgical duration exceeded 180 minutes. Additionally, one patient from Group F+S was excluded from the final statistical analysis because of block failure. In total, statistical analysis was conducted on 84 patients (Figure 2).
 |
Figure 2 Flow diagram of patient enrolment. There were no significant differences in sex, age, ASA classification, BMI, or duration of surgery among the three patients (Table 1). |
There were no statistically significant differences in gender, age, ASA classification, BMI, diagnosis, and duration of surgery among the three groups (Table 1).
 |
Table 1 Patient Characteristics and Intraoperative Data |
Primary Outcomes
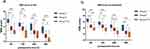
Compared with those of patients in Group C, the NRS scores of patients in Group F and F+S decreased at all postoperative time points during rest or activity (P<0.05); compared with those of patients in Group F, the NRS scores of patients in Group F+S decreased at all postoperative time points (P<0.05), as shown in Figure 3.
Secondary Outcomes
Compared with those of patients in Group C, the MAP and HR of patients in Groups F and F+S decreased at T2 (P<0.05) (Figure 4). Compared with that in Group C, the amount of sufentanil used during surgery was lower in Groups F and F+S (P<0.05); compared with that in Group F, the amount of sufentanil used during surgery was lower in Group F+S group (P<0.05). Moreover, the number of effective presses on the analgesic pump within 24 hours after surgery was lower in Groups F and F+S than in the C group (P<0.05). Additionally, compared with that in Group F, the number of effective presses on the analgesic pump within 24 hours after surgery decreased in Group F+S (P<0.05). The number of patients requiring rescue analgesia within 24 hours after surgery was lower in Group F+S than in Group C (P<0.05) (Table 2). In addition, the expression levels of IL-1β and TNF-α in patients in the C group were significantly greater at T3 and T4 than at T0, (P<0.05). At T3 and T4, the expression levels of IL-1β and TNF-α in patients in Groups F and F+S were significantly lower than those in Group C (P<0.05) (Figure 5).
 |
Table 2 Comparison of the Intraoperative Sufentanil Dosage and Postoperative Conditions |
Discussion
In this randomized trial, SFICB provided effective analgesia for total hip arthroplasty, particularly when combined with SNB, resulting in increased analgesic effects. It reduced intraoperative and postoperative opioid consumption, attenuated intraoperative hemodynamic fluctuations, and reduced the expression of inflammatory factors. Additionally, it decreased the incidence of postoperative nausea and vomiting.
The results of this study revealed that opioid consumption during surgery was lower in Group F group than in Group C, and that pain scores at 6 h, 12 h, 24 h, and 48 h after surgery were lower. This finding suggests the analgesic effectiveness of SFICB and is consistent with previous research findings.17–19 The sensory innervation of the hip includes the femoral nerve, obturator nerve, joint nerves of the sciatic nerve, branches of the sci nerve, and superior gluteal nerve.20 SFICB-targeted blockage of the femoral nerve, obturator nerve, and lateral femoral cutaneous nerve significantly reduces pain during hip joint surgery.21 Compared with FICB, SFICB has a more precise blockade effect on the lateral femoral cutaneous nerve. The lateral femoral cutaneous nerve supplies sensation to the skin on the outer side of the thigh, which is a surgical incision site for THA and a major site for early postoperative pain. Our research results indirectly reflect this feature. At the onset of surgery, within 5 minutes, patients in Group C presented significant increases in heart rate (HR) and mean arterial pressure (MAP), whereas patients in Groups F and F+S presented relatively stable haemodynamics. We believe that this may be due to the effective blockade of the lateral femoral cutaneous nerve by SFICB. SFICB can produce a strong blocking effect, which may be related to its anatomical factors. First, the femoral nerve and lateral femoral cutaneous nerve are located close to the proximal iliac spine, and operating above the inguinal ligament can simultaneously block these two nerves, resulting in a stronger blockade effect. Second, the lateral femoral cutaneous nerve often runs inside the anterior superior iliac spine, with significant individual variations in its course. Blocking above the inguinal ligament can increase the success rate of lateral femoral cutaneous nerve blockade.22 MR images have shown that during SFICB, local anaesthetics can more easily reach the femoral nerve and lateral femoral cutaneous nerve, producing a more complete block of sensation in the front and outer regions of the thigh23 and resulting in stronger analgesic effects than FICB.24 Additionally, the stronger analgesic effect of SFICB than FICB may also be related to targeted blockade of the obturator nerve.25 When SFICB is implemented, drugs can easily spread to the inner edge of the iliac muscle, improving the success rate of obturator nerve (ON) blockade.22,23 SFICB not only has a stronger blocking effect but is also easier to perform than the inguinal ligament below FICB, with clearer ultrasound imaging of tissue structures during the procedure and no difficulties in identifying tissue structures in elderly or obese patients.
Nevertheless, we also identified several limitations in our study. First, there are certain difficulties when SFICB is applied to thinner patients, as the greater size of the iliac spine may obstruct the direction of the needle. Moreover, for obese patients, the needle path under ultrasound guidance may be longer, which poses a challenge for beginners who are not proficient in ultrasound-guided nerve blockade. Our results also revealed that opioid consumption during surgery was lower in Group F+S than in Group F, resulting in less postoperative pain and fewer self-controlled analgesia requirements. Therefore, we believe that the implementation of SNB can provide more comprehensive analgesia. This is because the sciatic nerve and its branches are among the nerves that provide sensation to the hip joint.20 Additionally, the combination of SFICB with SNB can even further reduce postoperative rest and activity pain, aiding in the postoperative recovery of patients.
In the normal body, proinflammatory and anti-inflammatory factors are in a balanced state.26 However, surgical trauma can disrupt this balance and induce an inflammatory response.27 Compared with healthy individuals, elderly patients generally have reduced immune function prior to surgery, and due to the significant trauma associated with THA, surgery-induced trauma is more likely to induce the release of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), promoting the occurrence and progression of the inflammatory response.28,29 The levels of TNF-α and IL-1β are often positively correlated with the severity of the body’s inflammatory response.30 This study revealed varying degrees of increase in the levels of inflammatory factors in the three groups of patients, indicating that the body produces a large amount of proinflammatory cytokines in response to surgery. However, with nerve blockade intervention, the levels of IL-1β and TNF-α in Groups F and F+S were lower than those in Group C 30 minutes after surgery and at the end of surgery. This may be related to nerve blockade inhibiting the transmission of harmful stimuli to the spinal dorsal horn, suppressing the functional and structural remodelling of spinal dorsal horn neurons, and reducing the occurrence of the body’s inflammatory response.31,32 However, whether the extent of the reduction in the levels of these inflammatory factors has clinical significance still requires further investigation.
The results of this study showed that, compared with Group C, Groups F and F+S had smaller fluctuations in the MAP and HR at various time points, which may be related to the more comprehensive analgesia provided by nerve blockade. Furthermore, improved analgesia can lead to reduced use of opioid drugs, alleviate gastrointestinal reactions caused by opioids, and lower the incidence of postoperative nausea and vomiting. In particular, the combination of SFICB and SNB resulted in only one case of postoperative nausea and vomiting.
Limitations
This study has several limitations. First, it was a single-centre study with a small sample size, and subsequent research could increase the sample size to increase the experimental accuracy. Additionally, this study focused only on THA with a lateral approach, and further research is needed to determine whether this method can be applied to other surgeries. Finally, high-risk factors for postoperative nausea and vomiting also included a history of motion sickness, nonsmoking, and other factors that we did not record or statistically analyse preoperatively, which may have affected the incidence of postoperative nausea and vomiting.
Conclusion
The application of ultrasound-guided SFICB combined with SNB for total hip arthroplasty can provide more comprehensive analgesia, reduce postoperative NRS scores, alleviate haemodynamic fluctuations, decrease opioid drug use, and reduce the serum levels of inflammatory factors, especially when combined with SNB.
Data Sharing Statement
We are willing share all the individual participant data. All data used or analyzed in this study are available from the corresponding author (Lifeng Wang, [email protected]). It will be available following publication up on reasonable request and for 2 years.
Ethics Approval
The study was reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Gannan Medical University (LLSC-2021082401). All patients signed informed consent forms and were registered in the Chinese Clinical Trial Registry on August 26,2021 (Registration No. ChiCTR2100050375). This study conforms with the Helsinki Declaration.
Author Contributions
Jianshun Zhou and Mingling Guo are co-first authors. All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
Ganzhou Municipal Science and Technology Bureau (Program No. GZ2021ZSF074).
Disclosure
Lifeng Wang received grants from the Ganzhou Municipal Science and Technology Bureau (Program No. GZ2021ZSF074). None of the authors has a personal financial interest related to this trial.
References
1. Saul D, Riekenberg J, Ammon JC, Hoffmann DB, Sehmisch S. Hip Fractures: therapy, Timing, and Complication Spectrum. Orthop Surg. 2019;11(6):994–1002.
2. Taheriazam A, Saeidinia A, Keihanian F. Total Hip arthroplasty and cardiovascular complications: a review. Ther Clin Risk Manag. 2018;14:685–690. doi:10.2147/TCRM.S155918
3. Kumar P, Ksheersagar V, Aggarwal S, et al. Complications and mid to long term outcomes for Hip resurfacing versus total hip replacement: a systematic review and meta-analysis. Eur J Orthop Surg Traumatol. 2023;33(5):1495–1504. doi:10.1007/s00590-022-03361-5
4. Lanting BA, MacDonald SJ. The painful total Hip replacement: diagnosis and deliverance. Bone Joint J. 2013;95(11 Suppl A):70–73. doi:10.1302/0301-620X.95B11.32948
5. Richebé P, Capdevila X, Rivat C. Persistent Postsurgical Pain: pathophysiology and Preventative Pharmacologic Considerations. Anesthesiology. 2018;129(3):590–607. doi:10.1097/ALN.0000000000002238
6. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537–1546. doi:10.1016/S0140-6736(19)30352-6
7. Hofstede SN, Gademan MGJ, Vliet Vlieland TPM, et al. Preoperative predictors for outcomes after total Hip replacement in patients with osteoarthritis: a systematic review. BMC Musculoskelet Disord. 2016;17(1):212. doi:10.1186/s12891-016-1070-3
8. Wick EC, Grant MC, Wu CL. Postoperative Multimodal Analgesia Pain Management With Nonopioid Analgesics and Techniques: a Review. JAMA Surg. 2017;152(7):691–697. doi:10.1001/jamasurg.2017.0898
9. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of Postoperative Pain: a Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131–157. doi:10.1016/j.jpain.2015.12.008
10. Memtsoudis SG, Cozowicz C, Bekeris J, et al. Peripheral nerve block anesthesia/analgesia for patients undergoing primary Hip and knee arthroplasty: recommendations from the International Consensus on Anesthesia-Related Outcomes after Surgery (ICAROS) group based on a systematic review and meta-analysis of current literature. Reg Anesth Pain Med. 2021;46(11):971–985. doi:10.1136/rapm-2021-102750
11. Huang Z, Cai Y, Yang Y, et al. Effects of ultrasound-guided lumbar-sciatic nerve block and epidural anesthesia on the levels of IL-6, IL-8, TNF-α and coagulation factors in peripheral blood of elderly patients after Hip arthroplasty. J Med Biochem. 2022;41(4):433–440. doi:10.5937/jomb0-35847
12. Cardwell TW, Zabala V, Mineo J, et al. The Effects of Perioperative Peripheral Nerve Blocks on Peri- and Postoperative Opioid Use and Pain Management. Am Surg. 2022;88(12):2842–2850. doi:10.1177/00031348211023395
13. Okereke IC, Abdelmonem M. Fascia Iliaca Compartment Block for Hip Fractures: improving Clinical Practice by Audit. Cureus. 2021;13(9):e17836.
14. Jain N, Kotulski C, Al-Hilli A, et al. Fascia Iliaca Block in Hip and Femur Fractures to Reduce Opioid Use. J Emerg Med. 2022;63(1):1–9. doi:10.1016/j.jemermed.2022.04.018
15. Gola W, Bialka S, Owczarek AJ, et al. Effectiveness of Fascia Iliaca Compartment Block after Elective Total Hip Replacement: a Prospective, Randomized, Controlled Study. Int J Environ Res Public Health. 2021;18(9):4891. doi:10.3390/ijerph18094891
16. Bansal K, Sharma N, Singh MR, et al. Comparison of suprainguinal approach with infrainguinal approach of fascia iliaca compartment block for postoperative analgesia. Indian J Anaesth. 2022;66(Suppl 6):S294–s299. doi:10.4103/ija.ija_823_21
17. Wang YL, Liu Y-Q, Ni H, et al. Ultrasound-guided, direct suprainguinal injection for fascia iliaca block for total Hip arthroplasty: a retrospective study. World J Clin Cases. 2021;9(15):3567–3575. doi:10.12998/wjcc.v9.i15.3567
18. Castillón P, Veloso M, Gómez O, et al. Fascia iliaca block for pain control in Hip fracture patients. Rev Esp Cir Ortop Traumatol. 2017;61(6):383–389. doi:10.1016/j.recot.2017.07.004
19. Dulaney-Cripe E, Hadaway S, Bauman R, et al. A continuous infusion fascia iliaca compartment block in Hip fracture patients: a pilot study. J Clin Med Res. 2012;4(1):45–48. doi:10.4021/jocmr724w
20. Verbeek T, Adhikary S, Urman R, et al. The Application of Fascia Iliaca Compartment Block for Acute Pain Control of Hip Fracture and Surgery. Curr Pain Headache Rep. 2021;25(4):22. doi:10.1007/s11916-021-00940-9
21. Zhang L, Wang X-D, Qiu Y, et al. Ultrasound-guided fascia iliaca compartment block for pain control in total Hip arthroplasty: a systematic review and meta-analysis. Medicine. 2023;102(47):e36145. doi:10.1097/MD.0000000000036145
22. Zheng T, Hu B, Zheng C-Y, et al. Improvement of analgesic efficacy for total Hip arthroplasty by a modified ultrasound-guided supra-inguinal fascia iliaca compartment block. BMC Anesthesiology. 2021;21(1):75. doi:10.1186/s12871-021-01296-8
23. K Vermeylen, M Desmet, I Leunen, et al. Supra-inguinal injection for fascia iliaca compartment block results in more consistent spread towards the lumbar plexus than an infra-inguinal injection: a volunteer study. Reg Anesth Pain Med. 2019;44(1):483–491.
24. Bullock WM, Yalamuri SM, Gregory SH, et al. Ultrasound-Guided Suprainguinal Fascia Iliaca Technique Provides Benefit as an Analgesic Adjunct for Patients Undergoing Total Hip Arthroplasty. J Ultrasound Med. 2017;36(2):433–438. doi:10.7863/ultra.16.03012
25. Kumar K, Pandey RK, Bhalla AP, et al. Comparison of conventional infrainguinal versus modified proximal suprainguinal approach of Fascia Iliaca Compartment Block for postoperative analgesia in Total Hip Arthroplasty. A prospective randomized study. Acta Anaesthesiol Belg. 2015;66(3):95–100.
26. Liu S, Deng Z, Chen K, et al. Cartilage tissue engineering: from proinflammatory and anti‑inflammatory cytokines to osteoarthritis treatments (Review). Mol Med Rep. 2022;25(3). doi:10.3892/mmr.2022.12615.
27. Noah AM, Almghairbi D, Evley R, et al. Preoperative inflammatory mediators and postoperative delirium: systematic review and meta-analysis. Br J Anaesth. 2021;127(3):424–434. doi:10.1016/j.bja.2021.04.033
28. Blake Crabb E, Franco RL, Bowen MK, et al. G protein-coupled receptor kinase-2 in peripheral blood mononuclear cells following acute mental stress. Life Sci. 2016;145:184–189. doi:10.1016/j.lfs.2015.12.035
29. Ling XM, Fang F, Zhang X-G, et al. Effect of parecoxib combined with thoracic epidural analgesia on pain after thoracotomy. J Thorac Dis. 2016;8(5):880–887. doi:10.21037/jtd.2016.03.45
30. Uno M, Kitazato KT, Suzue A, et al. Inhibition of brain damage by edaravone, a free radical scavenger, can be monitored by plasma biomarkers that detect oxidative and astrocyte damage in patients with acute cerebral infarction. Free Radic Biol Med. 2005;39(8):1109–1116. doi:10.1016/j.freeradbiomed.2005.06.001
31. Mejía-Terrazas GE, Ruíz-Suárez M, Vadillo-Ortega F, et al. Effect of interscalene nerve block on the inflammatory response in shoulder surgery: a randomized trial. J Shoulder Elbow Surg. 2019;28(9):e291–e303. doi:10.1016/j.jse.2019.02.030
32. Kim J, Shim J-K, Song JW, et al. Postoperative Cognitive Dysfunction and the Change of Regional Cerebral Oxygen Saturation in Elderly Patients Undergoing Spinal Surgery. Anesth Analg. 2016;123(2):436–444. doi:10.1213/ANE.0000000000001352
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.