Back to Journals » Journal of Inflammation Research » Volume 17
Unleashing AdipoRon’s Potential: A Fresh Approach to Tackle Pseudomonas aeruginosa Infections in Bronchiectasis via Sphingosine Metabolism Modulation
Authors Xu JW , Chen FF, Qv YH, Sun CC, Zhang D, Guo Z, Wang YJ , Wang JF, Liu T, Dong L , Qi Q
Received 2 August 2024
Accepted for publication 18 October 2024
Published 24 October 2024 Volume 2024:17 Pages 7653—7674
DOI https://doi.org/10.2147/JIR.S483689
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Jia-wei Xu,1,2,* Fang-fang Chen,1,* Ying-hui Qv,1,* Cong-cong Sun,1 Dong Zhang,1,2 Zhi Guo,1 Yu-jiao Wang,3 Jun-fei Wang,4 Tian Liu,4 Liang Dong,1,2 Qian Qi1,2
1Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Shandong Institute of Respiratory Diseases, Shandong Characteristic Laboratory of Clinical Transformation of Respiratory Biological Immunity and Regenerative Medicine, Jinan, Shandong Province, 250014, People’s Republic of China; 2Department of Pulmonary and Critical Care Medicine, Shandong Provincial Qianfoshan Hospital, Shandong University, Jinan, Shandong Province, 250014, People’s Republic of China; 3Department of Clinical Laboratory Medicine, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Shandong Medicine and Health Key Laboratory of Laboratory Medicine, Jinan, Shandong Province, 250014, People’s Republic of China; 4Department of Pulmonary and Critical Care Medicine, Qilu Hospital, Shandong University, Jinan, Shandong Province, 250012, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qian Qi, Department of Pulmonary and Critical Care Medicine, the First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, #16766, Jingshi Road, Jinan City, Shandong Province, 250014, People’s Republic of China, Tel +86 13706380314, Email [email protected]
Purpose: Bronchiectasis patients are prone to Pseudomonas aeruginosa infection due to decreased level of sphingosine in airway. Adiponectin receptor agonist AdipoRon activates the intrinsic ceramidase activity of adiponectin receptor 1 (AdipoR1) and positively regulates sphingosine metabolism. This study aimed to investigate the potential therapeutic benefit of AdipoRon against Pseudomonas aeruginosa infection.
Methods: A mouse model of Pseudomonas aeruginosa lung infection and a co-culture model of human bronchial epithelial cells with Pseudomonas aeruginosa were established to explore the protective effect of AdipoRon. Liquid chromatography-mass spectrometry was used to detect the effect of AdipoRon on sphingosine level in lung of Pseudomonas aeruginosa-infected mouse models.
Results: The down-regulation of adiponectin and AdipoR1 in airway of bronchiectasis patients was linked to Pseudomonas aeruginosa infection. By activating AdipoR1, AdipoRon reduced Pseudomonas aeruginosa adherence on bronchial epithelial cells and protected cilia from damage in vitro. With the treatment of AdipoRon, the load of Pseudomonas aeruginosa in lung significantly decreased, and peribronchial inflammatory cell infiltration was lessened in vivo. The reduced level of sphingosine in the airway of Pseudomonas aeruginosa infected mice was replenished by AdipoRon, thus playing a protective role in the airway. Moreover, AdipoRon activated P-AMPKα/PGC1α, inhibited TLR4/P-NF-κB p65, and reduced expression of pro-apoptotic bax. However, the protective effect of AdipoRon on resisting Pseudomonas aeruginosa infection was weakened when AdipoR1 was knocked down.
Conclusion: AdipoRon protects bronchial epithelial cells and lung by enhancing their resistance to Pseudomonas aeruginosa infection. The mechanism might be modulating sphingosine metabolism and activating P-AMPKα/PGC1α while inhibiting TLR4/P-NF-κB p65.
Keywords: AdipoRon, Pseudomonas aeruginosa, bronchiectasis, AdipoR1, sphingosine metabolism
Introduction
Sphingolipid metabolism levels in the airway have been linked to the susceptibility to Pseudomonas aeruginosa infection.1 The accumulation of ceramide and a reduction of sphingosine level in tracheal and bronchial epithelial cells increase the infection susceptibility of cystic fibrosis mice and patients.2 Normalization of ceramide and sphingosine levels by inhalation of functional acid inhibitors prevents pulmonary Pseudomonas aeruginosa infection of cystic fibrosis mice.3 Reduced acid ceramidase activity impairs sphingosine metabolism, increasing the incidence of Pseudomonas aeruginosa infection in cystic fibrosis mice.4 Further studies have reported that the supplementation of recombinant acid ceramidase protein in cell and mouse models upregulates the sphingosine level and confers resistance to Pseudomonas aeruginosa infection.5 Our prior study found decreased sphingosine level in the lower respiratory tract of patients with non-cystic fibrosis bronchiectasis compared to the control group.6 We also established a negative association between sphingosine level and Pseudomonas aeruginosa infection.6 Moreover, sphingosine demonstrated antibacterial effect and inhibited the adherence of Pseudomonas aeruginosa to bronchial epithelial cells.6 It is indicated that patients with non-cystic fibrosis bronchiectasis are susceptible to Pseudomonas aeruginosa infection due to dysregulated sphingosine. Upstream regulators for maintaining sphingosine metabolism balance may be therapeutic approaches for tackling Pseudomonas aeruginosa infection in non-cystic fibrosis bronchiectasis.
The metabolic homeostasis of ceramide and sphingosine is regulated not only by acid ceramidase but also by other ceramidase, such as adiponectin receptor 1 (AdipoR1) and adiponectin receptor 2 (AdipoR2).7 AdipoR1 and AdipoR2 are activated by adiponectin to catalyse the hydrolysis of ceramide to produce sphingosine.8 Conversely, the deficiency of AdipoR1 leads to ceramide accumulation in retina in AdipoR1−/− mice.9 In human airway epithelial cells, AdipoR1 expression is predominant.10 In preterm infants, insufficient AdipoR1 expression during the canalicular and saccular stages of lung development raises the risk of bronchopulmonary dysplasia.11 When the AdipoR1 gene was knocked out, the bacterial infection of Listeria was worsened in mice.12 This indicates the crucial role of AdipoR1 in regulating the balance of ceramide and sphingosine metabolism and the protective effects of AdipoR1 on lung and bronchial epithelium to tackle Pseudomonas aeruginosa infection. Currently, no research is investigating whether the imbalance of sphingosine levels in the lower respiratory tract of patients with non-cystic fibrosis bronchiectasis is regulated by adiponectin and its receptor AdipoR1. We hypothesized that maintaining sphingosine metabolism balance by the supplementation of adiponectin and the activation of adiponectin receptor AdipoR1 might aid in resisting Pseudomonas aeruginosa infection in bronchiectasis.
The regulation of adiponectin gene expression is tightly controlled by a number of transcription factors.13 Nuclear transcription regulator peroxisome proliferators-activated receptor gamma (PPARγ) binds to exogenous lipid-like ligands and then interacts with specific PPAR response elements on nuclear DNA to modulate gene expression.14 PPARγ binds to the adiponectin promoter in mice and humans, serving as a major positive regulator of adiponectin gene expression.15 The deletion of PPARγ results in decreased levels of adiponectin.16 It is reported that agonising PPARγ inhibits Pseudomonas aeruginosa biofilm production in vitro,17 and PPARγ agonism decreases airway mucus hypersecretion in vivo.18 The attenuation of PPARγ impacts the innate immune function of primary airway epithelial cells by reducing expression of antimicrobial peptide and paraoxonase-2, as well as enhancing IL-8 expression.19 Previous study has revealed that non-cystic fibrosis bronchiectasis patients exhibit low expression of PPARγ in their airways, and PPARγ is inversely correlated with the presence of Pseudomonas aeruginosa.20 It is assumed that the decreased PPARγ expression attenuates airway epithelial host defense and increases susceptibility to Pseudomonas aeruginosa infection. Adiponectin reduces inflammation in various cells and increases macrophage migration through adiponectin-AdipoR1-PI3Kγ signaling pathway.21 Adiponectin exerts a variety of beneficial systemic effects through a sphingolipid-mediated pathway.7 There has no investigations of the relationship between PPARγ-adipoenctin-AdipoR1, sphingosine metabolism, and Pseudomonas aeruginosa infection in bronchiectasis patients. Importantly, downstream signaling pathways of AdipoR1 in Pseudomonas aeruginosa infection need to be further explored.
AdipoRon is a specific activator of AdipoR1 and AdipoR2, exerting protective effect against the damage of target organs through an improvement in lipid metabolism, as shown by the ratio of ceramide to sphingosine.22 The administration of AdipoRon can decrease the bacterial load of Listeria in the livers of adiponectin gene knockout mice and alleviate peritonitis.12 AdipoRon could be leveraged therapeutically in Pseudomonas aeruginosa infection of bronchiectasis patients. Therefore, the current study aims to explore the relationship between AdipoR1, sphingosine metabolism, and Pseudomonas aeruginosa infection in bronchiectasis patients; to determine the potential therapeutic benefit of AdipoR1 agonist AdipoRon against Pseudomonas aeruginosa infection; and to identify the role of AdipoR1 in sphingosine metabolism and its downstream signaling pathways in Pseudomonas aeruginosa infection.
Material and Methods
Bronchiectasis Patients’ Samples
Adiponectin Levels in Bronchoalveolar Lavage Fluid
This study was conducted in accordance with the Declaration of Helsinki. Bronchiectasis patients who underwent bronchoscopy from March 2021 to March 2022 in the First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital and Qilu Hospital of Shandong University were included. Inclusion criteria: (i) chest CT met the diagnostic criteria for bronchiectasis,6,23 (ii) aged 18–80 years, (iii) idiopathic or post-infection bronchiectasis, and (iv) no history of smoking. The control group consisted of individuals from these two hospitals, matched according to gender and age, who underwent bronchoscopy for unilateral peripheral pulmonary nodules (nodule diameter ≤ 3 cm) or hemoptysis without significant abnormality detected by chest CT (detailed inclusion and exclusion criteria were described in Supplementary Material). This study was approved by the First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital ethics committee (2021-S402) and Qilu Hospital of Shandong University ethics committee (2019–147), and informed consent from the patients was obtained prior to isolation and use of these samples. Bronchoalveolar lavage fluid (BALF) samples were collected to measure adiponectin levels using the enzyme-linked immunosorbent assay (ELISA) method (EK195 kit, MultiSciences, Hangzhou, China). Blank well and standard well with multiple dilutions were made as the control group.
Immunohistochemistry of Lung Tissue Specimens
Patients who underwent lobectomy for bronchiectasis, including bronchiectasis with recurrent refractory hemoptysis and limited bronchiectasis with chronic abscess formation, and were admitted to two hospitals from January 2021 to August 2022 were included; lung tissue specimens from bronchiectasis lesion sites were obtained. Inclusion criteria: CT of the chest met the diagnostic criteria of bronchiectasis,6,23 and pathological diagnosis of bronchiectasis was confirmed by pathologists (detailed inclusion and exclusion criteria were described in Supplementary Material). The control group included patients who were admitted to the thoracic surgery departments of both hospitals during the same period, were age- and gender-matched with the bronchiectasis group, and underwent lobectomy for isolated peripheral pulmonary nodules (≤3 cm in diameter); specimens were collected beyond 5 cm from the lung lesion. Immunohistochemistry was performed on paraffin sections of human lung tissue specimens using adiponectin receptor 1 (AdipoR1, MA5-32249, Thermo Fisher Scientific, USA) and PPARγ (#2435, Cell Signaling Technology, USA), with phosphate buffer saline (PBS) being the negative control.
Bacterial Culturing and Preparation of Bacterial Suspension
Pseudomonas aeruginosa PAO1 strain (ATCC) was provided by Dr. Yu-jiao Wang. Resuscitated bacteria from single colonies were inoculated into an LB liquid medium and cultured overnight at 37°C and 150 rpm/min for 16 hours. The sediment of bacterial solution was collected and resuspended in PBS. The bacterial concentration was determined by a McFarland turbidimetric method, and was adjusted to 10^8 colony-forming units (CFU)/mL. The prepared suspension was diluted 10-fold (original concentration, 10-fold, 10^2-fold, 10^3-fold, 10^4-fold, and 10^5-fold). Fifty μL of the diluted suspension were spread on LB agar plates, repeated three times. After 24-hour incubation at 37°C, CFU of the bacterial colonies were counted.
Experimental Cell Models
Human Bronchial Epithelial Cells Culture
Normal human primary bronchial epithelial cells were acquired from patients who underwent bronchoscopy because of unilateral peripheral pulmonary nodules (nodule diameter ≤3 cm) or hemoptysis without significant abnormalities on chest CT from December 2022 to September 2023 in the First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital (detailed inclusion and exclusion criteria were added in Supplementary Material). Under electron bronchoscopy, sterile protective brushes gently brushed 2–3 grade human primary bronchial epithelial cells.6 PneumaCultTM-Ex and PneumaCultTM-ALI culture mediums (05001and05008, STEMCELL Technologies, Canada) were used for air-liquid interface differentiation culture. Human bronchial epithelial cell line 16HBE (FH-1013) was purchased from FuHeng BioLogy (Hangzhou, China) and was cultured using KM medium (#2101, Shanghai Zhong Qiao Xin Zhou Biotechnology, Shanghai, China).
Pre-Treatment of Cell Models with AdipoRon
AdipoRon (HY-15848, MedChemExpress, USA) was prepared as a 100 mm stock solution in DMSO and diluted to the desired concentrations. It is reported that AdipoRon at concentrations of 5–50 μM activates AdipoR1 and increases adenosine 5’-monophosphate-activated protein kinase (AMPK) phosphorylation in a dose-dependent manner to almost the same extent as did adiponectin.24 Specifically, 10 μM, 50 μM, and 100 μM AdipoRon working solutions were added 24 hours before Pseudomonas aeruginosa infection. PBS and 0.1% DMSO solutions were used as control groups.
Co-Culture of Bronchial Epithelial Cell with Pseudomonas aeruginosa
Subsequently, 100 μL of bacterial suspension containing 0.5×10^5 CFU was added to the upper chamber of the transwell inserts of air-liquid interface cultured differentiated human bronchial epithelial cell for an 8-hour incubation. Preliminary results indicated a suitable bacterial concentration for co-culture with 16HBE cells and Pseudomonas aeruginosa was 0.5×10^4 CFU. Then 100 μL of bacterial suspension containing 0.5×10^4 CFU was added to the 16HBE cells culture medium and incubated for 8 hours.
Cell Transfection
Choose two siRNA sequences, AdipoR1 siRNA1 and siRNA2, to downregulate AdipoR1 expression. Plate 16HBE cells at 70–80% confluency in a 6-well plate. Transfect siRNA into 16HBE cells using Endo FectinTMMax transfection reagent (EF013, GeneCopoeia, Guangzhou, China) according to instructions. Replace the medium and continue culturing after 4–6 hours of transfection. Extract total RNA after 48 hours and protein after 72 hours of transfection. Validate the efficiency of AdipoR1 knockdown by performing quantitative real-time PCR (AG11706&11701 kit, AGbio, Changsha, China) and Western blot analysis.
Mouse Infection Model
Experimental Animals
C57BL/6 mice (specific-pathogen-free, 6–8 weeks old, female) were sourced from Jinan Pengyue Experimental Animal Breeding Co., Ltd. The mice, with consistent body size and weight, were used at around 8 weeks of age, exclusively using female mice. All animal experiments strictly adhered to the guidelines for laboratory animal care and use established by the National Institutes of Health, and were approved by the First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital Ethical Review Board for the Welfare of Laboratory Animals. Constant temperature and humidity feeding conditions were ensured. In order to minimize animal suffering during the study, it is necessary to master the experimental procedures and avoid repeated stimulation of mice.
Pseudomonas aeruginosa Lung Infection Mouse Model
Bacterial concentrations were verified by plating on LB agar and counting CFU. Before each experiment, the bacterial solution was washed twice and resuspended in PBS. Mice were anesthetized by intraperitoneal injection, being fixed on an animal shelf, and the tongue was pulled out to fully expose the glottis. When halogen cold light source was used to irradiate the pharynx and larynx of mice, 35 μL of Pseudomonas aeruginosa (2×106 CFU) in PBS solution was slowly injected into the lung to establish the mouse infection model.25
Groups and Dosing
C57BL/6 mice were acclimated for one week and then divided into five groups. Each group consisted of at least six mice. The dose of AdipoRon was chosen based on previous studies which reported that intravenous injection of AdipoRon (50 mg/kg body weight) significantly activate AMPK in skeletal muscle and liver,24 and that injection of AdipoRon (5 or 12.5 mg/kg, iv) significantly attenuated arterial thrombosis.26 Treatment of the five groups: (i) Uninfected control: mice were anesthetized by intraperitoneal injection and instilled with 35 μL of PBS via the airway; (ii) Pseudomonas aeruginosa infection with DMSO control: 8 hours prior, mice were given an intravenous injection of DMSO solution as a control; (iii) Pseudomonas aeruginosa infection with low-dose AdipoRon: 8 hours prior, mice were treated with AdipoRon (5 mg/kg, intravenous injection); (iv) Pseudomonas aeruginosa infection with high-dose AdipoRon: 8 hours prior, mice were treated with AdipoRon (50 mg/kg, intravenous injection); and (v) Pseudomonas aeruginosa infection with adiponectin Acrp30 recombinant protein: 4 hours prior, mice were treated with adiponectin Acrp30 recombinant protein (HY-P7358, MedChemExpress, USA, 1 mg/kg, intravenous injection) as described.27 Subsequently, mice in 4 groups (except for the uninfected control group) were anesthetized by intraperitoneal injection and infected with 35 μL of Pseudomonas aeruginosa (2×106 CFU) in PBS solution via the airway. After 24 hours of infection, mice were euthanized and specimens were collected. Bacterial CFU counts were performed on the lung homogenates of mice in each group.
Metabonomics of Lung Tissue in Mouse Model
Liquid chromatography-tandem mass spectrometry (LC-MS) (UPLC-TripleTOF system, AB SCIEX) was used to analyze the metabolomics of lung tissue. For different groups of mouse model, samples were uniform and the residual body fluid was quickly absorbed by the non-dusting absorbent paper. The tissue was cut into 100 mg pieces, accurately weighed and packed according to the same amount. There were 6 replicates in each group.
Immunofluorescence and Western Blot
Immunofluorescence and Western blot were performed to compare the expression levels of various markers. These markers included AdipoR1 (MA5-32249, Thermo Fisher Scientific, USA), adiponectin (ab22554, AbCam, UK), PPARγ (#2435, Cell Signaling Technology, USA), ZO-1 (21,773-1-AP, ProteinTech, Wuhan, China), TLR4 (A5258, ABclonal, Wuhan, China), PGC1α (A12348, ABclonal, Wuhan, China), α-tubulin (ab7291, AbCam, UK), P-AMPKα (Thr172) (#2535, Cell Signaling Technology, USA), AMPKα (#5832, Cell Signaling Technology, USA), P-NF-κB p65 (#3033, Cell Signaling Technology, USA), NF-κB p65 (ET1603-12, HUABIO, Hangzhou, China), bcl2 (ET1702-53, HUABIO, Hangzhou, China), bax (ET1603-34, HUABIO, Hangzhou, China), GAPDH (SA30-01, HUABIO, Hangzhou, China) and β-actin (M1210-2, HUABIO, Hangzhou, China). The expression level of the Pseudomonas aeruginosa antibody (PA1-73116, Thermo Fisher Scientific, USA) was detected.
Hematoxylin and Eosin (HE) Staining
The membrane at the bottom of the transwell chamber, containing differentiated human bronchial epithelial cells from different treatment groups cultured in an air-liquid interface, was excised and used to prepare frozen sections for HE staining. HE staining was also performed on mouse lung tissue specimens from different treatment groups. A semi-quantitative grading method of inflammation score was used to evaluate the severity of peribronchial inflammatory cell infiltration: 0, normal; 1, few cells; 2, a ring of inflammatory cells 1 cell layer deep; 3, a ring of inflammatory cells 2–4 cells deep; 4, a ring of inflammatory cells of > 4 cells deep.28 The researchers who evaluated the inflammation score of HE staining were blinded to the grouping information.
Scanning Electron Microscopy
Different groups’ transwell chamber membranes were fixed in electron microscopy fixative. The membranes were then rinsed with 0.1 mL phosphate buffer, fixed with 1% osmium tetroxide for 2–4 hours, washed with double-distilled water, dehydrated in ethanol gradients, dried under vacuum with tert-butanol, and finally observed and recorded using a German ZEISS Sigma 300 field emission scanning electron microscope after ion sputtering.
TUNEL Assay
A one-step TUNEL cell apoptosis detection kit (KTA2011, Abbkine, Wuhan, China) was used. The slides were sealed and observed under a confocal laser scanning microscope (Nikon A1+, Japan). For each stained section, six random fields were selected to calculate the total number of cells and the number of positively stained cells. The apoptotic rate was calculated as (number of apoptotic cells / total number of cells) × 100%.
Statistical Analysis
All experiments mentioned above have undergone a minimum of three independent repetitions for experimental validation. Metric data with a normal distribution were represented using the eqn method, and a t-test was employed for comparing two groups. Multiple group comparisons were performed using a one-way analysis of variance, followed by pairwise comparisons using the least significant difference test. The Mann–Whitney U-test was used to compare the two groups with uneven variance. Categorical data was described using rates or proportions, and comparisons between two or more groups were conducted using the χ2 test. The metabolites with variable important in projection (VIP) > 1 and P<0.05 were screened. Principal component analysis (PCA) were used to compare the differences among the groups. Statistical analysis was performed using SPSS 25.0 software, and graphical representation was done using GraphPad Prism 8.0 medical software. A significance level of P<0.05 was considered statistically significant.
Results
The Decreasing PPARγ/Adiponectin/AdipoR1 in Bronchiectasis Was Associated with Pseudomonas aeruginosa Infection
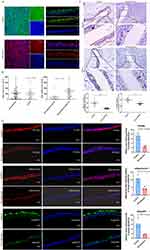
The Human Protein Atlas database assessed AdipoR1 and AdipoR2 protein expression in lung. AdipoR1, not AdipoR2, predominated in lung tissue. Immunofluorescence confirmed the expression of AdipoR1 and adiponectin in ciliary surface of differentiated human primary bronchial epithelial cells (Figure 1A). Age, sex, and body mass index did not differ between 47 cases of bronchiectasis patients and 22 control group patients (Table 1). Adiponectin levels in BALF showed no significant distinction between bronchiectasis and control groups, yet BALF adiponectin expression was lower in bronchiectasis patients with Pseudomonas aeruginosa-positive cultures compared to those with negative cultures (Figure 1B).
 |
Table 1 Comparison of the General Data Between Patients with Bronchiectasis and the Control Group |
Immunohistochemical analysis of lung tissue samples revealed reduced expression of PPARγ and AdipoR1 in bronchiectasis patients’ airway epithelium compared to controls (Figure 1C). Air-liquid interface differentiated human primary bronchial epithelial cells exposed to Pseudomonas aeruginosa exhibited decreased expression of PPARγ, adiponectin, and AdipoR1 (Figure 1D).
The expression of AdipoR1 in the airway epithelium of the mouse model infected with Pseudomonas aeruginosa were significantly decreased by immunofluorescence analysis and Western blot (Figure 2A–C). With the treatment of AdipoRon or adiponectin Acrp30 recombinant protein, the expression level of AdipoR1 in the airway epithelium of the Pseudomonas aeruginosa infected mice increased (Figure 2D).
The above results strongly suggested that the down-regulation of PPARγ, adiponectin, and AdipoR1 in bronchiectasis patients was linked to Pseudomonas aeruginosa infection. The next step was to explore the role of AdipoRon in protecting bronchial epithelium and lung against Pseudomonas aeruginosa in vitro and in vivo.
AdipoRon Activated AdipoR1, Reduced Bacterial Adherence on Bronchial Epithelial Cells and Protected Cilia from Damage
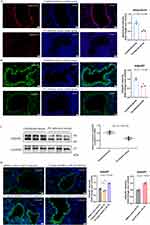
Cell models were established by using the human primary bronchial epithelial cells from donors (donors’ information see Table S1). With the treatment of AdipoRon, the expression level of AdipoR1 in air-liquid interface differentiated human primary bronchial epithelial cells increased (Figure 3A–D). Immunofluorescence and Western blot results indicated that 100 μM AdipoRon significantly enhanced the expression level of AdipoR1 (Figure 3A–D).
There was a great number of Pseudomonas aeruginosa adhered on the air-liquid differentiated human primary bronchial epithelial cell surface both in the PBS control group and the DMSO control group (Figure 3E). By contrast, the amount of bacteria adhered on the cells in the 100 μM AdipoRon group significantly reduced (Figure 3E).
Scanning electron microscopy revealed that the 50 μM and 100 μM AdipoRon could both reduce bacteria-induced damage to cilia (Figure 3F). Furthermore, AdipoRon was found to increase the expression level of the tight junction protein ZO-1 between cells, with the 100 μM AdipoRon showing the most significant effect (Figure 3G).
AdipoRon Reduced the Load of Pseudomonas aeruginosa and Peribronchial Inflammatory Cell Infiltration of Mice
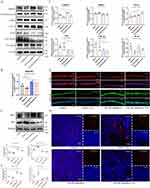
Compared to the uninfected group, the Pseudomonas aeruginosa-infected model group exhibited increased infiltration of inflammatory cells around the bronchial (Figure 4A). A semi-quantitative grading of inflammation was used to evaluate the severity of peribronchial inflammatory cell infiltration. The inflammatory score in the 50mg/kg AdipoRon treatment group and Acrp30 recombinant protein treatment group were significantly lower than that in the DMSO control group (1.8 ± 0.3 vs 3.8 ± 0.2; 1.3 ± 0.2 vs 3.8 ± 0.2) (Figure 4A and B).
The CFU of Pseudomonas aeruginosa in the lung tissue homogenates in the 50 mg/kg AdipoRon treatment group and Acrp30 recombinant protein treatment group were significantly lower than that in the DMSO control group [(1.8 ± 0.3)×10^4 CFU vs (439.4 ± 20.4)×10^4 CFU; (14.2 ± 0.3)×10^4 CFU vs (439.4 ± 20.4)×10^4 CFU] (Figure 4C and D). The immunofluorescence also showed the reduced load of Pseudomonas aeruginosa in the airway of mice in 50 mg/kg AdipoRon treatment group and Acrp30 recombinant protein treatment group (Figure 4E).
Overall, AdipoRon protected bronchial epithelium and lung against Pseudomonas aeruginosa infection in vitro and in vivo. We next explored the mechanism of the protective effects of AdipoRon.
AdipoRon Increased Sphingosine Level in the Lung of Mouse Infection Model
The levels of sphingosine in Pseudomonas aeruginosa infected mice were significantly lower than that in uninfected mice (Figure 5A). The metabolites in the lung tissue of mice between 50 mg/kg AdipoRon treatment group, 5 mg/kg AdipoRon treatment group and the DMSO control group were significantly different (Figure 5B). With the treatment of 50 mg/kg AdipoRon, the sphingosine levels in the lung tissue of mice significantly increased (Figure 5C).
The metabolites in the lung tissue of mice in adiponectin Acrp30 recombinant protein treatment group were significantly different from the DMSO control group (Figure 5D). With the treatment of Acrp30 recombinant protein, the sphingosine levels in the lung tissue of mice also significantly increased (Figure 5E).
In brief, the reduced level of sphingosine in the airway of mice caused by Pseudomonas aeruginosa infection can be replenished by AdipoRon, thus playing a protective role in the airway.
AdipoRon Activated P-AMPKα/PGC1α, Inhibited TLR4/P-NF-κB p65, and Reduced Expression of Bax Both in vivo and in vitro
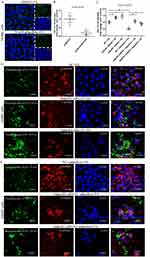
With the treatment of AdipoRon, the expression level of AdipoR1 in Pseudomonas aeruginosa-infected mice significantly increased (Figure 6A and B). AdipoRon treatment rescued the decrease of AMPK phosphorylation, and improved the expression level of PGC1α (Figure 6A and B). Moreover, AdipoRon inhibited the expression levels of TLR4/P-NF-κB p65 and reduced the expression of pro-apoptotic bax (Figure 6A and B). Similarly, the expression level of AdipoR1 in the adiponectin Acrp30 recombinant protein treatment group increased (Figure 6C and D). The adiponectin Acrp30 recombinant protein raised the expression level of P-AMPKα and PGC1α, inhibited the expression level of TLR4 and P-NF-κB p65, and reduced the expression of bax (Figure 6C and D).
According to the preliminary finding that 100 μM AdipoRon effectively reduces Pseudomonas aeruginosa adherence to human bronchial epithelial cells, the mechanism of the protective effects of AdipoRon was explored in Pseudomonas aeruginosa-infected cell models. Infection of Pseudomonas aeruginosa led to a decrease in AdipoR1 expression in air-liquid differentiated human primary bronchial epithelial cells, while the addition of AdipoRon increased the expression of AdipoR1 (Figure 7A and B). AdipoRon promoted the expression of P-AMPKα/PGC1α and inhibited the expression of TLR4/P-NF-κB p65 (Figure 7A, C and D). In addition, AdipoRon treatment reduced the expression of pro-apoptotic bax in Pseudomonas aeruginosa-infected cell models (Figure 7E). TUNEL staining revealed the increased bronchial epithelial cell apoptosis following Pseudomonas aeruginosa infection, but AdipoRon reduced cell apoptosis (Figure 7F and G).
Knockdown of AdipoR1 and Its Effects on Bacterial Adherence
Both 50 μM and 100 μM AdipoRon increased the expression level of AdipoR1 in 16HBE cells (Figure 8A and B). Knockdown of AdipoR1 by siRNA partly inhibited the ability of AdipoRon to activate AMPK phosphorylation and the ability of AdipoRon to improve the expression of PGC1α (Figure 8C–F). The inhibitory effect of AdipoRon on TLR4/P-NF-κB p65 was also weakened when AdipoR1 was knocked down (Figure 8G and H). Additionally, the expression of pro-apoptotic bax increased after AdipoR1 was knocked down (Figure 8I and J).
Compared to the DMSO control group, adding 50 μM AdipoRon reduced the surface-adhered bacterial load of Pseudomonas aeruginosa on 16HBE cells (Figure 9A and B). However, knockdown of AdipoR1 expression resulted in a significant increase in the surface-adhered bacterial load of Pseudomonas aeruginosa on 16HBE cells (Figure 9C and D). Following the knockdown of AdipoR1, adding AdipoRon did not reduce the surface-adhered bacterial load on 16HBE cells (Figure 9E).
Collectively, the protective effect of AdipoRon on resisting Pseudomonas aeruginosa infection was weakened when AdipoR1 was knocked down.
Discussion
This study employed a bronchial epithelium-Pseudomonas aeruginosa co-culture model and a Pseudomonas aeruginosa-infected mouse model to confirm the significantly reduced Pseudomonas aeruginosa adhesion to bronchial epithelial cells and the decreased bacterial load in lung tissue following treatment with AdipoRon. Mechanisms potentially involved the activation of AdipoR1 to positively regulate sphingosine metabolism, to up-regulate the expression of P-AMPKα/PGC1α, to inhibit the expression of TLR4/P-NF-κB p65, and to suppress the pro-apoptotic bax expression, ultimately safeguarding lung and bronchial epithelium against Pseudomonas aeruginosa infection (Figure 10). AdipoR1 assumes a critical role in lung protection.
Pseudomonas aeruginosa infection accelerates the progression of bronchiectasis in patients. Targeted eradication treatment or protection against Pseudomonas aeruginosa infection may be crucial in delaying disease progression.29 Previous studies have shown that AdipoRon can reduce the CFU of Listeria monocytogenes in the mouse liver, protecting the liver from Listeria monocytogenes infection.12 Consistently, this study confirmed that AdipoRon reduced Pseudomonas aeruginosa adherence to human bronchial epithelial cells in vitro and decreased the load of Pseudomonas aeruginosa in mouse models, indicating its ability to protect bronchial epithelium and lung against Pseudomonas aeruginosa infection. Sphingosine itself possesses antibacterial properties by making pores on bacterial membrane,30 and directly disrupting the bacterial cell wall and membrane.6 This study verified that sphingosine levels in the airway decreased in Pseudomonas aeruginosa-infected mouse models, while the levels of sphingosine increased significantly after AdipoRon treatment, thus play their own antibacterial effect. There is a strong association between the supplement of AdipoRon, the positively regulated sphingosine metabolism and reduced Pseudomonas aeruginosa infection in vitro and in vivo. Previous study has determined that the protective mechanism of AdipoRon on target organs against damage is related to maintaining normal levels of sphingosine metabolism.31 Hence, these results suggest that increasing sphingosine levels by the treatment of AdipoRon aids in resisting Pseudomonas aeruginosa infection.
Adiponectin has been studied as a safeguard of lung due to its anti-inflammatory and anti-apoptotic properties through activating adiponectin receptors.13,32,33 However, injection of recombinant adiponectin is not attractive due to its relatively high plasma concentrations, short half-life, and its complex quaternary structure.34 By contrast, AdipoRon can activate AdipoRs without affecting the systemic adiponectin level.35 The core mechanism of AdipoRon resisting against Pseudomonas aeruginosa infection is the activation of adiponectin receptor AdipoR1 and AdipoR2. Given the low expression of AdipoR2 in bronchial epithelial cells, the role of AdipoR2 was not addressed. There is currently no research investigating the protective role of AdipoR1 in bronchial epithelium and lung against Pseudomonas aeruginosa infection. This study showed that with the increasing expression of AdipoR1, the Pseudomonas aeruginosa infections were weakened both in vitro and in vivo. On the contrary, when the expression level of AdipoR1 was knocked down, the amount of Pseudomonas aeruginosa adhered to the surface of bronchial epithelium cells significantly increased. Unfortunately, the expression of AdipoR1 in airway reduced in bronchiectasis patients, and the decreasing expression of AdipoR1 was related to Pseudomonas aeruginosa infection. Therefore, the novel finding of the protective role of AdipoR1 might uncover new therapeutic targets for treating bronchiectasis. However, there are many differences between the mouse model and human bronchiectasis. Regarding the in vitro nature of the experiments, more experimental evidences are needed.
It was interesting that with the increasing expression level of AdipoR1, the expressions of P-AMPKα and PGC1α were improved in both Pseudomonas aeruginosa-infected cell models and mouse models. Previous studies have reported that adiponectin activates the SIRT3 transcription by triggering the AdipoR1/P-AMPK/PGC1α pathway, leading to the enrichment of SIRT3 in mitochondria, thereby exerting a neuroprotective effect on neurons.36 Adiponectin ameliorates lung ischemia-reperfusion injury through SIRT1-PINK1 signaling-mediated mitophagy.37 Pseudomonas aeruginosa and its quorum-sensing molecules can elude host defense by impairing host epithelial mitochondria.38 Regrettably, there is limited investigation into AdipoRon’s impact on mitochondrial function through the AdipoR1/P-AMPK/PGC1α pathway in our study. Further mechanistic studies are needed to explore the involvement of mitochondrial function in the protective effects of AdipoRon against Pseudomonas aeruginosa infection. Likewise, after the treatment of AdipoRon, the severity of peribronchial inflammatory cell infiltration was reduced and the expression of pro-inflammatory TLR4/P-NF-κB and pro-apoptotic bax was decreased. But the broader implications of these pathways for inflammation and apoptosis control was not explored in this study. The potential anti-inflammatory and anti-apoptotic effects of AdipoRon need to be dressed in further studies, especially in chronic Pseudomonas aeruginosa infection models. Our study results suggest that AdipoRon may be a promising drug to treat Pseudomonas aeruginosa infection. However, AdipoRon is limited by its poor solubility and bioavailability.39 Developing higher-affinity agonists with improved pharmacokinetics with a tissue- or cell-specific delivery approach should be pursued in the future. Moreover, AdipoRon can bypass the blood-brain barrier, indicating its direct effect on the brain.40 Therefore, long-term effects and potential side effects need to be observed in the mouse model.
It is concluded that the main mechanisms by which AdipoRon exerts its protective effect on bronchial epithelium and lung resisting Pseudomonas aeruginosa infection are the activated AdipoR1 and the balanced sphingosine metabolism. Hence, understanding the mechanism behind down-regulation expression of AdipoR1 and insufficient sphingosine metabolism in airway of bronchiectasis patients could uncover new therapeutic targets for treating bronchiectasis. This study observed a significant reduction in BALF adiponectin levels in bronchiectasis patients infected with Pseudomonas aeruginosa. Furthermore, both cell models and mouse models of Pseudomonas aeruginosa infection confirmed the decreased expression of adiponectin and AdipoR1 post-infection. PPARγ functions as an upstream regulatory factor of adiponectin, and down-regulation or loss of PPARγ expression will result in decreased adiponectin levels.19 The quorum-sensing molecule 3O-C12-HSL, produced by Pseudomonas aeruginosa PAO1 strain, diminishes PPARγ expression in bronchial epithelial cells.17 It is hypothesized that the down-regulated PPARγ-adiponectin-AdipoR1 expression intensifies Pseudomonas aeruginosa infection and the expression of PPARγ is further down-regulated after infection, leading to a detrimental cycle. The down-regulation of airway epithelial PPARγ expression in cystic fibrosis patients is associated with defective differentiation of airway basal cells.41 However, the underlying mechanisms responsible for the down-regulation of PPARγ and AdipoR1 in bronchiectasis patients remain unknown. The mechanism of insufficient sphingosine metabolism in the airway of bronchiectasis patients was lack of in-depth study. Further investigations are needed to explore the potential mechanisms.
This study confirms AdipoRon’s protective role on bronchial epithelial cells and lung by enhancing their resistance to Pseudomonas aeruginosa infection. However, given the preliminary nature of some results, further mechanistic investigations are necessary to address the remaining limitations. Firstly, the role of 1-phosphorylated sphingosine (S1P) in AdipoRon’s signaling pathways has not been fully explored in our study. Sphingosine is converted to S1P by sphingosine kinase.42 S1P plays a crucial role in maintaining the integrity and homeostasis of the alveolar epithelial barrier.43 Adiponectin receptors’ activation may increase the level of sphingosine and promote the formation of the pro-survival factor S1P. Further research is needed to ascertain the involvement of S1P in the mechanisms underlying AdipoRon’s protective effects on bronchial epithelial cells against Pseudomonas aeruginosa infection. Secondly, this study did not investigate the impact of AdipoRon on mitochondrial function. Exploring these pathways could deepen the understanding of how AdipoRon confers its protective effects. Thirdly, mouse models of chronic Pseudomonas aeruginosa infection were lacking in this study. Clinical strains of Pseudomonas aeruginosa were not used in this study. Constructing a model of chronic Pseudomonas aeruginosa infection, especially using clinical strains, might help clarify long-term responses to AdipoRon and sphingosine regulation, making the study more relevant to clinical bronchiectasis cases. Fourthly, measuring adiponectin receptor levels in the BALF samples would be more helpful, as adiponectin could also come from edema. Fifthly, AdipoR2 was not addressed in the study. It is reported that AdipoR1, rather than AdipoR2, mediated the protective effects of adiponectin/AdipoR signaling against oxidative stress and mitochondrial damage.36 Although AdipoR2 is rarely expressed in the bronchial epithelium,9 it is also possible that AdipoRon activates AdipoR2. To completely eliminate the involvement of AdipoR2 receptor, it’s necessary to consider AdipoR2 knockout model in further studies. Moreover, understanding the interplay between these receptors could clarify the broader protective effects of AdipoRon. Finally, given the in vitro nature of the experiments and the potential differences between the mouse model and human bronchiectasis, more researches are needed.
Conclusion
In summary, AdipoRon protects bronchial epithelial cells and lung by enhancing their resistance to Pseudomonas aeruginosa infection. The mechanism might be modulating sphingosine metabolism and activating P-AMPKα/PGC1α while inhibiting TLR4/P-NF-κB p65.
Abbreviation
AdipoR1, adiponectin receptor 1; AdipoR2, adiponectin receptor 2; PPARγ, peroxisome proliferators-activated receptor gamma; BALF, bronchoalveolar lavage fluid; ELISA, enzyme-linked immunosorbent assay; PBS, phosphate buffer saline; CFU, colony-forming units; AMPK, adenosine 5’-monophosphate-activated protein kinase; LC-MS, liquid chromatography- tandem mass spectrometry; HE, hematoxylin and eosin; VIP, variable important in projection; PCA, principal component analysis; S1P, 1-phosphorylated sphingosine.
Ethics Approval and Informed Consent
This study was approved by the First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital ethics committee (ethics review number: 2021-S402) and Qilu Hospital of Shandong University ethics committee (ethics review number: 2019-147), and all study participants provided informed consent. This study was approved by the First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital Ethical Review Board for the Welfare of Laboratory Animals (QFSYYPZ20230203).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants from National Natural Science Foundation of China (82100056), Natural Science Foundation of Shandong Province (ZR2024MH153; ZR2021QH170), Natural Science Foundation of Shandong Province (ZR2022QH235), Natural Science Foundation of Shandong Province (ZR2022QH078), National Natural Science Foundation of China (82000025), National Natural Science Foundation of China (82000031) and Natural Science Foundation of Shandong Province (ZR2020QH002).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Ebenezer DL, Berdyshev EV, Bronova IA, et al. Pseudomonas aeruginosa stimulates nuclear sphingosine-1-phosphate generation and epigenetic regulation of lung inflammatory injury. Thorax. 2019;74(6):579–591. doi:10.1136/thoraxjnl-2018-212378
2. Gardner AI, Wu Y, Verhaegh R, et al. Interferon regulatory factor 8 regulates expression of acid ceramidase and infection susceptibility in cystic fibrosis. J Biol Chem. 2021;296:100650. doi:10.1016/j.jbc.2021.100650
3. Becker KA, Riethmüller J, Seitz AP, et al. Sphingolipids as targets for inhalation treatment of cystic fibrosis. Adv Drug Deliv Rev. 2018;133:66–75. doi:10.1016/j.addr.2018.04.015
4. Becker KA, Verhaegh R, Verhasselt HL, et al. Acid ceramidase rescues cystic fibrosis mice from pulmonary infections. Infect Immun. 2021;89(2):e00677–20. doi:10.1128/IAI.00677-20
5. Gardner AI, Haq IJ, Simpson AJ, et al. Recombinant acid ceramidase reduces inflammation and infection in cystic fibrosis. Am J Respir Crit Care Med. 2020;202(8):1133–1145. doi:10.1164/rccm.202001-0180OC
6. Qi Q, Xu J, Wang Y, et al. Decreased sphingosine due to down-regulation of acid ceramidase expression in airway of bronchiectasis patients: a potential contributor to Pseudomonas aeruginosa infection. Infect Drug Resist. 2023;16:2573–2588. doi:10.2147/IDR.S407335
7. Holland WL, Miller RA, Wang ZV, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17(1):55–63. doi:10.1038/nm.2277
8. Vasiliauskaité-Brooks I, Sounier R, Rochaix P, et al. Structural insights into adiponectin receptors suggest ceramidase activity. Nature. 2017;544(7648):120–123. doi:10.1038/nature21714
9. Lewandowski D, Foik AT, Smidak R, et al. Inhibition of ceramide accumulation in AdipoR1-/- mice increases photoreceptor survival and improves vision. JCI Insight. 2022;7(4):e156301. doi:10.1172/jci.insight.156301
10. Miller M, Cho JY, Pham A, et al. Adiponectin and functional adiponectin receptor 1 are expressed by airway epithelial cells in chronic obstructive pulmonary disease. J Immunol. 2009;182(1):684–691. doi:10.4049/jimmunol.182.1.684
11. Kang NY, Ivanovska J, Tamir-Hostovsky L, et al. Chronic intermittent hypoxia in premature infants: the link between low fat stores, adiponectin receptor signaling and lung injury. Adv Exp Med Biol. 2018;1071:151–157.
12. Masamoto Y, Arai S, Sato T, et al. Adiponectin enhances antibacterial activity of hematopoietic cells by suppressing bone marrow inflammation. Immunity. 2016;44(6):1422–1433. doi:10.1016/j.immuni.2016.05.010
13. Fang H, Judd RL. Adiponectin regulation and function. Compr Physiol. 2018;8(3):1031–1063.
14. Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454(7203):470–477. doi:10.1038/nature07202
15. He W, Barak Y, Hevener A, et al. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100(26):15712–15717. doi:10.1073/pnas.2536828100
16. Maeda N, Takahashi M, Funahashi T, et al. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50(9):2094–2099. doi:10.2337/diabetes.50.9.2094
17. Bedi B, Maurice NM, Ciavatta VT, et al. Peroxisome proliferator-activated receptor-γ agonists attenuate biofilm formation by Pseudomonas aeruginosa. FASEB J. 2017;31(8):3608–3621. doi:10.1096/fj.201700075R
18. Liu DS, Liu WJ, Chen L, et al. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, attenuates acrolein-induced airway mucus hypersecretion in rats. Toxicology. 2009;260(1–3):112–119. doi:10.1016/j.tox.2009.03.016
19. Bedi B, Lin KC, Maurice NM, et al. UPR modulation of host immunity by Pseudomonas aeruginosa in cystic fibrosis. Clin Sci. 2020;134(14):1911–1934. doi:10.1042/CS20200066
20. Burr LD, Rogers GB, Chen AC, et al. PPARγ is reduced in the airways of non-CF bronchiectasis subjects and is inversely correlated with Pseudomonas aeruginosa. PLoS One. 2018;13(8):e0202296. doi:10.1371/journal.pone.0202296
21. Jin X, Wang Y. Mechanisms of adiponectin in regulation of proinflammatory cytokine production and migration in macrophages. J Inflamm Res. 2021;14:981–993. doi:10.2147/JIR.S292137
22. Choi SR, Lim JH, Kim MY, et al. Adiponectin receptor agonist AdipoRon decreased ceramide and lipotoxicity and ameliorated diabetic nephropathy. Metabolism. 2018;85:348–360. doi:10.1016/j.metabol.2018.02.004
23. Flume PA, Chalmers JD, Olivier KN. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet Lond Engl. 2018;392(10150):880–890. doi:10.1016/S0140-6736(18)31767-7
24. Okada-Iwabu M, Yamauchi T, Iwabu M, et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013;503(7477):493–499. doi:10.1038/nature12656
25. Liu Y, Xie YZ, Shi YH, et al. Airway acidification impaired host defense against Pseudomonas aeruginosa infection by promoting type 1 interferon β response. Emerg Microbes Infect. 2022;11(1):2132–2146. doi:10.1080/22221751.2022.2110524
26. Zhou XH, Cheng ZP, Lu M, et al. Adiponectin receptor agonist AdipoRon modulates human and mouse platelet function. Acta Pharmacol Sin. 2023;44(2):356–366. doi:10.1038/s41401-022-00943-1
27. Ivanovska J, Kang NC, Ivanovski N, et al. Recombinant adiponectin protects the newborn rat lung from lipopolysaccharide-induced inflammatory injury. Physiol Rep. 2020;8(17):e14553. doi:10.14814/phy2.14553
28. Myou S, Leff AR, Myo S, et al. Blockade of inflammation and airway hyperresponsiveness in immune-sensitized mice by dominant-negative phosphoinositide 3-kinase-TAT. J Exp Med. 2003;198(10):1573–1582. doi:10.1084/jem.20030298
29. Chai YH, Xu JF. How does Pseudomonas aeruginosa affect the progression of bronchiectasis? Clin Microbiol Infect. 2020;26(3):313–318. doi:10.1016/j.cmi.2019.07.010
30. Verhaegh R, Becker KA, Edwards MJ, et al. Sphingosine kills bacteria by binding to cardiolipin. J Biol Chem. 2020;295(22):7686–7696. doi:10.1074/jbc.RA119.012325
31. Kim Y, Lim JH, Kim EN, et al. Adiponectin receptor agonist ameliorates cardiac lipotoxicity via enhancing ceramide metabolism in type 2 diabetic mice. Cell Death Dis. 2022;13(3):282. doi:10.1038/s41419-022-04726-8
32. Sun Y, Zhao D, Yang Y, et al. Adiponectin exerts cardioprotection against ischemia/reperfusion injury partially via calreticulin mediated anti-apoptotic and anti-oxidative actions. Apoptosis. 2017;22(1):108–117. doi:10.1007/s10495-016-1304-8
33. Yao R, Cao Y, He YR, et al. Adiponectin attenuates lung fibroblast activation and pulmonary fibrosis induced by paraquat. PLoS One. 2015;10(5):e0125169. doi:10.1371/journal.pone.0125169
34. Pajvani UB, Hawkins M, Combs TP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279(13):12152–12162. doi:10.1074/jbc.M311113200
35. Kim Y, Park CW. Mechanisms of adiponectin action: implication of adiponectin receptor agonism in diabetic kidney disease. Int J Mol Sci. 2019;20(7):1782. doi:10.3390/ijms20071782
36. Zhang S, Wu X, Wang J, et al. Adiponectin/AdiopR1 signaling prevents mitochondrial dysfunction and oxidative injury after traumatic brain injury in a SIRT3 dependent manner. Redox Biol. 2022;54:102390. doi:10.1016/j.redox.2022.102390
37. Jiang T, Liu T, Deng X, et al. Adiponectin ameliorates lung ischemia-reperfusion injury through SIRT1-PINK1 signaling- mediated mitophagy in type 2 diabetic rats. Respir Res. 2021;22(1):258. doi:10.1186/s12931-021-01855-0
38. Di Martino S, Tardia P, Cilibrasi V, et al. Lead optimization of benzoxazolone carboxamides as orally bioavailable and CNS penetrant acid ceramidase inhibitors. J Med Chem. 2020;63(7):3634–3664. doi:10.1021/acs.jmedchem.9b02004
39. Onodera T, Ghazvini Zadeh E, Xu P, et al. PEGylated AdipoRon derivatives improve glucose and lipid metabolism under insulinopenic and high-fat diet conditions. J Lipid Res. 2021;62:100095. doi:10.1016/j.jlr.2021.100095
40. Formolo DA, Lee TH, Yau SY. Increasing adiponergic system activity as a potential treatment for depressive disorders. Mol Neurobiol. 2019;56(12):7966–7976. doi:10.1007/s12035-019-01644-3
41. Bou Saab J, Bacchetta M, Chanson M. Ineffective correction of PPARγ signaling in cystic fibrosis airway epithelial cells undergoing repair. Int J Biochem Cell Biol. 2016;78:361–369. doi:10.1016/j.biocel.2016.07.035
42. Hadas Y, Vincek AS, Youssef E, et al. Altering sphingolipid metabolism attenuates cell death and inflammatory response after myocardial infarction. Circulation. 2020;141(11):916–930. doi:10.1161/CIRCULATIONAHA.119.041882
43. Chen Q, Rehman J, Chan M, et al. Angiocrine sphingosine-1-phosphate activation of S1PR2-YAP signaling axis in alveolar type II cells is essential for lung repair. Cell Rep. 2020;31(13):107828. doi:10.1016/j.celrep.2020.107828
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Decreased Sphingosine Due to Down-Regulation of Acid Ceramidase Expression in Airway of Bronchiectasis Patients: A Potential Contributor to Pseudomonas aeruginosa Infection
Qi Q, Xu J, Wang Y, Zhang J, Gao M, Li Y, Dong L
Infection and Drug Resistance 2023, 16:2573-2588
Published Date: 28 April 2023