Back to Journals » Journal of Pain Research » Volume 17
Upregulation of NGF/TrkA-Related Proteins in Dorsal Root Ganglion of Paclitaxel-Induced Peripheral Neuropathy Animal Model
Authors Kim Y , Je MA, Jeong M , Kwon H , Jang A, Kim J, Choi GE
Received 25 March 2024
Accepted for publication 25 October 2024
Published 20 November 2024 Volume 2024:17 Pages 3919—3932
DOI https://doi.org/10.2147/JPR.S470671
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Wendy Imlach
Yeeun Kim,1,2,* Min-A Je,1,2,* Myeongguk Jeong,1,2 Hyeokjin Kwon,1,2 Aelee Jang,3 Jungho Kim,1,2 Go-Eun Choi1,2
1Department of Clinical Laboratory Science, College of Health Sciences, Catholic University of Pusan, Busan, 46252, Republic of Korea; 2Next-Generation Industrial Field-Based Specialist Program for Molecular Diagnostics, Brain Busan 21 Plus Project, Graduate School, Catholic University of Pusan, Busan, 46252, Republic of Korea; 3Department of Nursing, University of Ulsan, Ulsan, 44610, Republic of Korea
*These authors contributed equally to this work
Correspondence: Go-Eun Choi; Jungho Kim, Department of Clinical Laboratory Science, College of Health Sciences, Catholic University of Pusan, Busan, 46252, Republic of Korea, Tel +82-51-510-0563 ; +82-51-510-0660, Fax +82-51-510-0568, Email [email protected]; [email protected]
Background: Paclitaxel (PTX) can induce chemotherapy-induced peripheral neuropathy (CIPN) as a side effect. The aim of this study was to understand the neurochemical changes induced by NGF/TrkA signaling in PTX-induced neuropathic pain.
Methods: The PTX-induced CIPN mouse model was evaluated using nerve conduction velocity (NCV) and behavioral tests. Protein expression in mouse DRG was observed by Western blotting and immunohistochemistry. Nerve growth factor (NGF), IL-6, and IL-1β mRNA levels were determined using qRT-PCR by isolating total RNA from whole blood.
Results: PTX showed low amplitude and high latency values in NCV in mice, and induced cold allodynia and thermal hyperalgesia in behavioral assessment. Activating transcription factor 3 (ATF3) and MAPK pathway related proteins (ERK1/2), tropomyosin receptor kinase A (TrkA), calcitonin gene related peptide (CGRP) and transient receptor potential vanilloid 1 (TRPV1) were upregulated 7th and 14th days after 2 mg/kg and 10 mg/kg of PTX administration. Protein kinase C (PKC) was upregulated 7th days after 10 mg/kg PTX treatment and 14th days after 2 mg/kg and 10 mg/kg PTX administration. NGF, IL-6, and IL-1β fold change values also showed a time- and dose-dependent increase.
Conclusion: Taken together, our findings may improve our understanding of the nociceptive symptoms associated with PTX-induced neuropathic pain and lead to the development of new treatments for peripheral neuropathy.
Keywords: Chemotherapy-induced peripheral neuropathy, paclitaxel, nerve growth factor, tropomyosin receptor kinase A, transient receptor potential vanilloid 1
Introduction
Chemotherapy-induced peripheral neuropathy can occur with the administration of several chemotherapeutic drugs, such as cisplatin, oxaliplatin, vincristine, and paclitaxel (PTX). PTX is widely used to treat several types of cancer, including breast, ovarian, and lung cancers, but it reduces the quality of life of chemotherapy patients as a major side effect.1,2 The likelihood of peripheral neuropathy caused by PTX depends on the cumulative dose, the dose per cycle, and clinical symptoms, such as hypoesthesia, paresthesia, burning pain, tingling, mechanical allodynia, and distal extremity pain.1,3–7 Animal models of PTX-induced peripheral neuropathy have been developed to explain the mechanisms of PTX-induced pain.8–11
Nerve growth factor (NGF) is a neurotrophin that is crucial for neuronal survival and development.12 Recently, increased NGF expression was observed in an animal model of neuropathic pain with a neuropathic pain component.13 In animal models of neuropathy, NGF levels increased in the dorsal root ganglion (DRG), dorsal horn neurons and peripheral nerves.14–16 NGF binds to tropomyosin receptor kinase A (TrkA), a receptor with high affinity for NGF, triggering pain signals by stimulating the production of several peptides that promote pain transmission.17 NGF allows the recognition of NGF receptors by intracellular signaling proteins.18 NGF plays a central role in hyperalgesia, acting through its receptor TrkA to initiate hyperalgesia in a TRPV1-dependent manner.19,20 NGF/TrkA signaling mainly bypasses the TRPV1 pathway, which releases calcitonin gene-related peptide (CGRP) and directs it to the noninvasive membrane.
Transient receptor potential vanilloid 1 (TRPV1) is a cation channel expressed in primary sensory neurons and plays an important role in hyperalgesia.21–23 It is present in polymodal nociceptors and can be sensitized by protons and endogenous compounds released after tissue damage.21 While its role as an indicator of thermal nociception is widely recognized, evidence suggests that TRPV1 may additionally contribute to mechanotransduction at both peripheral and central levels, particularly after nerve injury.18–20,24,25 In particular, a recent study reported that TRPV1 expressed in the spinal cord was associated with chemotherapy-induced neuropathic injury.23,26 CGRP is a biomarker for a neuropeptide subpopulation (approximately 40% of cells) consisting primarily of small neurons with unmyelinated axons (C-fibers) that innervate polymodal nociceptors.27 TRPV1 activation releases sensory neuropeptides such as CGRP from nerve endings, inducing nerve depolarization and resulting in pain. Neuropeptides are known to enhance nociceptor sensitization and activate effector cell receptors.28
The aim of this study was to understand the neurochemical changes induced by NGF/TrkA signaling in PTX-induced neuropathic pain. Therefore, we aimed to observe changes in the activation of CGRP and TRPV1 in the DRG in an animal model of PTX-induced neuropathic pain and confirm the effect of PTX administration on the frequency of CGRP in immune-labeled DRG neurons. Taken together, our findings may improve our understanding of the nociceptive symptoms associated with PTX-induced neuropathic pain and lead to the development of new treatments for peripheral neuropathy.
Material and Methods
Animals
Male BALB/c mice, aged four weeks and weighing 20–25 g, were obtained from Samtaco-Bio (Samtaco-Bio, Gyeonggi-do, Republic of Korea). After a one-week acclimatization period, the experiments were conducted. The animals were housed under a 12/12h light/dark cycle at a constant ambient temperature of 20 ± 1°C with humidity maintained at 55 ± 5%. The animal experimental procedures were approved by the Animal Research Ethics Committee (CUP AEC 2023–003) of the Catholic University of Pusan, and complied with the ethical guidelines of the National Institutes of Health and the International Association for the Study of Pain.
Drug Administration
Mice weighing approximately 20–25 g were randomly divided into four groups with six mice per group. To maintain social housing, three mice were housed per cage. PTX (6 mg/mL) was dissolved in sterile saline containing 50% Kolliphor EL (Sigma-Aldrich, MO, USA) and 50% ethanol (Sigma-Aldrich, MO, USA) to obtain a final concentration of 1 mg/mL. Mice were administered the diluted drug intraperitoneally (i.p). at a dose of 10 mg/kg every other day for 14 days. Similarly, after diluting PTX to a dose of 2 mg/kg, groups of mice (n = 6) were injected intraperitoneally. This dosing schedule has been used in previous studies to induce CIPN in mice.29–32 The vehicle group received treatment for 14 days with sterile, endotoxin-free saline. AR786, a TrkA inhibitor (Array Biopharma, CO, USA), was used in BALB/c mice (n = 6).33–35 A stock solution of 750 mg/mL AR786 in DMSO was used. The dosing schedule used in this study was repeated three times.
Behavioral Assessment
The response duration (s) for the cold plate test, and the incubation period (m/s) for the hot plate test were measured and monitored 14 days after the administration of PTX (10 mg/kg), PTX (2 mg/kg), and oral AR786. Six mice per group were placed on cooling plates (Panlab SL, Barcelona, Spain), and the temperature was adjusted to 4 °C. The time taken for the mouse to lick its paw was recorded, and the animal was immediately removed from the cooling plate. A 60-second blocking period was maintained to prevent foot damage. To measure the hot plate test, the surface temperature of the hot plate apparatus (Ugo Basile, VA, Italy) was set to 52 °C. The reaction latency and the latency (s) between the time that the mouse was placed and the start of paw-licking behavior were monitored at 2-day intervals. A 40-second blocking time was used to minimize tissue damage.36 All behavioral assessments were performed individually.
Electrophysiology
Before sacrifice, the nerve conduction velocity (NCV) in mice was measured using a Medtronic Keypoint Portable 2-channel electromyography machine (Medtronic, Skovlunde, Denmark). All mice were anesthetized with alfaxalone (25 mg/kg; Careside, Seongnam, Republic of Korea) and xylazine (5 mg/kg; Bayer Korea, Seoul, Republic of Korea) and gently dissected to expose the sciatic nerve and posterior gastrocnemius muscle from surrounding tissues for NCV measurement. Stimulating needle electrodes were placed on the proximal and distal sides of the sciatic nerve, and recording needle electrodes were inserted into the gastrocnemius on the same side. The NCV was obtained by calculating the amplitude (mV) and latency (s) between the stimulation and recording electrodes.37
Histological Observation
After sacrificing the mice, the sciatic nerve and gastrocnemius muscle were dissected and fixed in 10% formalin (Sigma-Aldrich, MO, USA). The tissues were then embedded in paraffin, sectioned, and stained with haematoxylin and eosin at St. Maria Pathology (Busan, Republic of Korea).
Immunohistochemistry
For immunohistochemistry (IHC), the sections were incubated with primary antibodies, namely anti-Activating transcription factor 3 (ATF3) (1:100; Abcam, MA, USA) and anti-Calcitonin gene-related peptide (CGRP) (1:1000; Abcam, MA, USA) overnight at 4°C. They were then incubated with a goat anti-rabbit secondary antibody for 1 hour at room temperature. After antigen retrieval, the sections were stained using the VECTASTAIN Elite ABC HRP kit (Vector Laboratories, CA, USA) according to the manufacturer’s instructions. Finally, all paraffin sections were counterstained with haematoxylin and eosin and mounted using mounting medium. Morphological changes were photographed using a Leica DMi1 inverted microscope (Leica Microsystems, Wetzlar, Germany).
Western Blotting
Proteins were extracted from mouse DRG samples using radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitors, and homogenized using the IKA Homogenizer (LKLAB, Gyeonggi-do, Republic of Korea). The following antibodies were used for Western blot analysis: TrkA (cat no. 2505; 1:1000; Cell Signaling Technology, MA, USA), PKC (cat no. P5704; 1:1000; Sigma-Aldrich, MO, USA), TRPV1 (cat no. sc-398417; 1:100; Santa Cruz Biotechnology, CA, USA), CGRP (cat no. 14959; 1:1000; Cell Signaling Technology, MA, USA), and ERK1/2 (44/42) (cat no. 9102; 1:1000; Cell Signaling Technology, MA, USA), as well as GAPDH (cat no. 2118; 1:3000; Cell Signaling Technology, MA, USA). The target proteins were detected using horseradish peroxidase (HRP)-labeled anti-mouse IgG (cat no. 7076; Cell Signaling Technology, MA, USA) or anti-rabbit IgG secondary antibodies (cat no. 7074; Cell Signaling Technology, MA, USA), and the images were analyzed using the Molecular Image Gel Doc™ XR+ system (Bio-Rad, CA, USA).
Total RNA Extraction from Whole Blood in Mice
Total RNA was isolated from whole blood using the Mouse RiboPure™-Blood RNA Isolation Kit (Thermo Fisher Scientific, MA, USA). RNA yield was quantified by measuring UV absorbance using a NanoDrop spectrophotometer (Thermo Fisher Scientific, MA, USA). Total RNA was stored at −80°C until cDNA synthesis was performed.
Quantitative Real-Time Polymerase Chain Reaction (PCR) Analysis
To measure gene expression levels, we synthesized complementary DNA (cDNA) using the PrimeScript™ RT Master Mix (Takara Korea Biomedical, Seoul, Republic of Korea). Quantitative PCR was performed using a QuantStudio 7 Flex Real-Time PCR system (Thermo Fisher Scientific, MA, USA) and SYBR Green Realtime PCR MasterMix (TOYOBO, Osaka, Japan). Macrogen (Seoul, Republic of Korea) provided the primers to detect the expression and sequence of IL-1β, IL-6, and NGF in mouse blood. Reaction conditions for real-time PCR were as follows: 95 °C for 10 minutes, followed by 40 cycles of 95 °C for 10 seconds and 60 °C for 30 seconds. We used the Comparative Cycle Threshold (CT) method to analyze the real-time PCR data with GAPDH as a control. All experiments were performed in triplicate, and the final data were analyzed using the 2-ΔΔCT method.
Statistical Analysis
All results were performed in triplicate and shown as mean ± standard deviation (SD). Statistical analysis was performed using GraphPad Prism 6 software (GraphPad Software Inc., CA, USA) version 6.01. Data were analyzed using two-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test. Values with p < 0.05 were considered statistically significant.
Results
Behavioral Alterations
Mechanical allodynia is a hallmark of mouse CIPN.38,39 Locomotor activity tests were performed to assess potential motor deficits induced by PTX. Hind paw licking was measured in response to the cold plate test twice a week for 14 days (2nd, 4th, 6th, 8th, 10th, 12th and 14th days). The shaking duration of mice was significantly increased, indicating the development of cold allodynia. As shown in (Figure 1A), significant cold allodynia was observed from days 2 to 14 after PTX administration (p < 0.001; 2 mg/kg vs vehicle, 2nd, 4th, 6th, 8th, 10th, 12th and 14th days), (p < 0.001; 10 mg/kg vs vehicle, 2nd, 4th, 6th, 8th, 10th, 12th and 14th days). The PTX 10 mg/kg + AR786 treatment group normalized (p < 0.001; PTX 10 mg/kg vs PTX 10 mg/kg + AR786, 2nd, 4th, 6th, 8th, 10th, 12th and 14th days). Significant thermal hyperalgesia was observed in response to the hot plate test from the 4th to the 14th day after PTX treatment initiation (Figure 1B) (p < 0.001; 2 mg/kg vs vehicle, 4th, 6th, 8th, 10th, 12th and 14th days), (p < 0.001; 10 mg/kg vs vehicle, 4th, 6th, 8th, 10th, 12th and 14th days). The PTX 10 mg/kg + AR786 treatment group normalized (p < 0.001, PTX 10 mg/kg vs PTX 10 mg/kg + AR786, 4th, 6th, 8th, 10th, 12th and 14th days). Our results demonstrated that 2 mg/kg and 10 mg/kg PTX induced sensory neuropathy with mechanical allodynia and distinct gait changes. In contrast, the 10 mg/kg PTX + AR786 group recovered to a level similar to that of the vehicle.
Electrophysiology
The NCV analysis was performed to further characterize the peripheral neuropathy of the sciatic nerve. Stimulation of the sciatic nerve in anesthetized mice showed changes in NCV over time. All animals treated with PTX showed a significant decrease in amplitude (mV) compared to the vehicle on the 7th day (p < 0.05; 2 mg/kg PTX vs vehicle, p < 0.01; 10 mg/kg PTX vs vehicle) and on the 14th day (p < 0.01; 10 mg/kg PTX vs vehicle) (Figure 2A). The amplitude decreased proportionally with the PTX dose. Latency values decreased in animals treated with PTX 2 mg/kg (p < 0.01; vs vehicle) and PTX 10 mg/kg (p < 0.001; vs vehicle) after 14 days. However, no significant changes were observed on the 7th day (see Figure 2B). In summary, PTX treatment consistently decreased the NCV amplitude (mV) and increased latency (s) in a dose-dependent manner, indicating axonal pathology.40–42
Muscle Atrophy
This study confirms the presence of muscle atrophy in the PTX group compared to the vehicle and PTX 10 mg/kg + AR786 groups. Visible gastrocnemius atrophy was observed in the PTX 2 mg/kg and PTX 10 mg/kg groups. Gastrocnemius muscles were collected and weighed after sacrifice each week, and as the cumulative drug concentration of PTX (2 mg/kg or 10 mg/kg) increased over time, the muscle weight decreased due to muscle atrophy. Significant muscle mass loss due to gastrocnemius atrophy was observed between the 4th and 14th days (p < 0.05; 2 mg/kg vs vehicle, day 6th), (p < 0.001; 2 mg/kg vs vehicle, 8th, 10th, 12th and 14th days), (p < 0.01; 10 mg/kg vs vehicle, 4th day) and (p < 0.001; 10 mg/kg vs vehicle, 6th, 8th, 10th, 12th and 14th days). However, the PTX 10 mg/kg + AR786 treatment group had normalized muscle mass (p < 0.01; 10 mg/kg vs PTX 10 mg/kg + AR786, 4th day) and (p < 0.001; 10 mg/kg vs PTX 10 mg/kg + AR786, 6th, 8th, 10th, 12th and 14th days) (Figure 3A). Increased fibrosis and fat infiltration, which are obvious features of muscular atrophy, were confirmed through haematoxylin and eosin staining. Photomicrographs (100x) were obtained for each mouse (Figure 3B). Degenerated muscle fibers were quantified using Image J and the vehicle group was compared with the PTX group, the 7th day (p < 0.001; 2 mg/kg vs vehicle), (p < 0.001; 10 mg/kg vs vehicle), (p < 0.001; 10 mg/kg vs PTX 10 mg/kg + AR786) and 14th day (p < 0.001; 2 mg/kg vs vehicle), (p < 0.001; 10 mg/kg vs vehicle), (p < 0.001; 10 mg/kg vs PTX 10 mg/kg + AR786) after administration (Figure 3C). Measurements of muscle fiber diameter showed smaller muscle fibers in the rectus femoris muscle of the PTX-treated group than in the vehicle group (Figure 3B), suggesting reduced muscle mass and muscular atrophy in the PTX-treated group.
Increased ATF3 Expression in and in DRG Cells After PTX Administration
The expression of ATF3 is a sensitive indicator of neuronal damage or injury.43 Immunohistochemical (IHC) staining showed increased expression of ATF3 in the dorsal root ganglia (DRG) of mice treated with PTX (Figure 4A). Significant changes were observed on the 7th and 14th days following PTX administration. On the 7th and 14th days, there was a significant increase in ATF3 expression for both PTX 2 mg/kg vs vehicle (p < 0.001) and PTX 10 mg/kg vs vehicle (p < 0.001) groups. Normalization in the PTX 10 mg/kg + AR786 treatment group on the 7th and 14th days showed significant changes (p < 0.001; PTX 10 mg/kg vs PTX 10 mg/kg + AR786) (Figure 4B and C).
Expression of ERK1/2 in Mouse DRG After Paclitaxel Treatment
The modulation of MAPK activation, including the ERK1/2, p38 kinase, and JNK pathways, has been associated with pain development.44,45 In this study, Western blot analysis was conducted to measure ERK1/2 expression in the DRG of mice treated with PTX and compared with that of vehicle-treated mice (Figure 5A). In the PTX group, ERK1/2 expression was upregulated on the 7th day (p < 0.01; PTX 2 mg/kg vs vehicle, p < 0.001; PTX 10 mg/kg vs vehicle) and 14th day (p < 0.001; PTX 10 mg/kg vs vehicle) after PTX administration. The PTX 10 mg/kg + AR786 group showed a decrease in ERK1/2 expression similar to that of the vehicle group on the 14th day (p < 0.001; PTX 10 mg/kg vs PTX 10 mg/kg + AR786) (Figure 5B). The Western blot original image of ERK1/2 is included in the supplemental material (Figure S1).
Furthermore, this study analyzed the mRNA expression of IL-1β in the whole blood, which showed a significant increase in mice treated with 10 mg/kg PTX on the 14th day after cumulative administration of PTX (p < 0.01; vs vehicle) (Figure 5C). The IL-6 fold-change values in whole blood showed a statistically significant increase in the PTX 10 mg/kg group (p < 0.05; vs vehicle) (Figure 5D).
PTX Injection Induced Expression of NGF/TrkA, PKC, TRPV1 and CGRP Proteins in CIPN Mouse DRGs
NGF, its receptor TrkA, and related downstream proteins (PKC, TRPV1, and CGRP) were significantly elevated on the 7th and 14th days after PTX administration. NGF signaling through the TrkA receptor is involved in the basic mechanisms of neuropathic pain.13 Compared with the vehicle group, NGF fold-change values significantly increased on the 7th (p < 0.01; PTX 2 mg/kg vs vehicle, p < 0.01; PTX 10 mg/kg vs vehicle) and 14th (p < 0.001; PTX 2 mg/kg vs vehicle, p < 0.05; PTX 10 mg/kg vs vehicle) days with PTX treatment (Figure 6A). Based on the NGF data, other potential downstream signals were analysed by Western blotting (Figure 6B). TrkA, PKC, TRPV1, and CGRP proteins were strongly expressed in mouse DRG on the 7th and 14th days of PTX administration compared with the vehicle group. TrkA (p < 0.05; 2 mg/kg vs vehicle, p < 0.001; 10 mg/kg vs vehicle, 7th day) and (p < 0.05; 2 mg/kg vs vehicle, p < 0.001; 10 mg/kg vs vehicle, 14th day), PKC (p < 0.001; 10 mg/kg vs vehicle, 7th day) and (p < 0.05; 2 mg/kg vs vehicle, p < 0.001; 10 mg/kg vs vehicle, 14th day), TRPV1 (p < 0.05; 2 mg/kg vs vehicle, p < 0.001; 10 mg/kg vs vehicle, 7th day) and (p < 0.01; 2 mg/kg vs vehicle, p < 0.001; 10 mg/kg vs vehicle, 14th day), and CGRP (p < 0.05; 2 mg/kg vs vehicle, p < 0.001; 10 mg/kg vs vehicle, 7th day) and (p < 0.01; 2 mg/kg vs vehicle, p < 0.001; 10 mg/kg vs vehicle, 14th day). For PTX 10 mg/kg + AR786, the expression levels were close to those of the vehicle (Figure 6C). The original images of the Western blot of TrkA, PKC, TRPV1, and CGRP are included in the supplemental material (Figure S2).
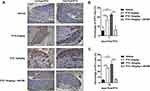
CGRP Expression in CIPN Mouse Spinal Cord
As a result of IHC, CGRP protein showed strong yellow or brown staining of cell nuclei in the PTX 2mg/kg group and the PTX 10mg/kg group compared to the vehicle group days 14th after PTX injection (Figure 7A). As a result of quantitative analysis, there was little CGRP-positive expression in the vehicle group. Comparison with the vehicle group, the PTX group showed significant expression levels (p < 0.001; PTX 2mg/kg vs vehicle), (p < 0.001; PTX 10mg/kg vs vehicle). In the PTX 10mg/kg + AR786 treatment group, it was normalized to the vehicle level (p < 0.001; PTX 10mg/kg+ AR786 vs PTX 10mg/kg) (Figure 7B).
Discussion
PTX-induced peripheral neuropathic pain is a major problem for cancer patients, often leading to complaints of pain in the hands and feet from mechanical and thermal stimulation, accompanied by magnetic pain and tingling. This type of pain can severely reduce patients’ quality of life and may lead to treatment discontinuation.40,46 It has been reported that CIPN caused by administration of anticancer drugs causes damage to the sciatic nerve as well as DRG.47,48
Therefore, in this paper, we observed changes in mice days 7th and 14th after administration of 2 mg/kg or 10 mg/kg PTX to investigate the pathogenesis and potential treatment of peripheral neuropathic pain induced by PTX. It was confirmed that thermal hyperalgesia and cold allodynia were observed in both mice 7th and 14th days after administration of 2 mg/kg or 10 mg/kg PTX. The NCV showed low amplitude values and high latency values over time. These results showed that PTX treatment induced CIPN. The results of cold allodynia / thermal hyperalgesia and NCV test in rats are consistent with previous reports.49,50 In addition, muscular atrophy and weakness have been reported as symptoms accompanying PTX administration. They tended to report the most severe acute side effects.51 In this study, changes in muscle wasting and muscle weakness in mice caused by PTX administration were confirmed.
In this study, we focused on the NGF/TrkA mechanism, one of the pain signaling mechanisms, and added an experimental group in which AR786, a TrkA antagonist, was administered simultaneously with PTX in all experiments. First, an increase in the mRNA expression of NGF was confirmed of the CIPN mouse model administered with PTX, and activation of the TrkA receptor and a significant increase in the expression of ERK1/2 and PKC, which are sub-signaling mechanisms, in mouse DRG were confirmed, respectively. In addition, it was confirmed that the expression of representative pain signal molecules, TRPV1 and CGRP, increased due to the activation of the TrkA receptor. These results were not confirmed in the experimental group in which AR786 and PTX were simultaneously administered, and it was demonstrated that the signal transduction mechanism was induced by the activity of the TrkA receptor.
Following TrkA receptor activation, the signaling mechanisms include the Ras-MAPK, PI3K and PLC-γ1 pathways.13 Several studies have shown that MAPK pathways including (c-Jun NH2 terminal kinase) JNK, (extracellular signal-regulated kinase) ERK1/2 and p38 contribute to CIPN and play a central role in chemotherapy-induced peripheral neuropathy pain. In this study, it was confirmed that ERK1/2 in DRG tissue was activated by PTX treatment. The JNK and p38 pathways will require further studies to confirm more accurate signaling mechanisms. Moreover, it was confirmed that PTX treatment released pro-inflammatory cytokines by mRNA expression of IL-1β and IL-6 and cytokines in whole blood. It has been reported that these pro-inflammatory cytokines are involved in additional sensitization of peripheral sensory neurons leading to allodynia.52,53
In immunohistochemical analysis, we showed increased ATF3 protein levels in PTX-treated mouse DRG. Previous studies have reported that PTX produces up-regulation of ATF3 in the primary sensory neurons of DRG of experimental animals and is also induced in microglia and macrophages by the cytokine IL-6.43,54,55
In nociceptive neurons, NGF plays an important role in pain and represents its initiation. Re-efflux of NGF and activation of TrkA, a tyrosine kinase that binds to NGF receptors, is induced by inflammation. NGF-binding induces TrkA dimerization, autophosphorylation and subsequent binding and activation of PLCγ.56,57 In this study, increased PKC expression was observed in the DRG of the CIPN mouse model. This finding provides a potential mechanism and target by which persistent activation of PKC may contribute to CIPN hyperalgesia.38
The TRPV1 receptor is reported to an important play in the regulation of nociceptive signal transduction in inflammatory pain and is also reported to play a pivotal role in peripheral neuropathic pain induced by chemotherapy drugs.41,42,58 In this study, it was confirmed that the TRPV1 receptor in DRG tissue was activated by PTX treatment. In addition, we observed increased CGRP expression in DRG tissues of PTX-treated mice. In the experimental group administered with AR786 and PTX, neither TRPV1 receptor nor CGRP expression increased. These results demonstrate that the response is driven by TrkA receptor activation. A previous study reported that intrathecal (i.t). administration of K252a, a nonselective inhibitor of several receptor tyrosine kinases, including the TrkA receptor, significantly alleviated mechanical hypersensitivity in paclitaxel-treated rats.59 In our study, we demonstrated that NGF directly targets TrkA by using AR786, a highly specific inhibitor of TrkA, and confirmed the expression of factors such as ERK1/2, PKC, TRPV1, and CGRP to confirm the pain pathway in paclitaxel-induced peripheral neuropathy. Activation of TRPV1 can lead to neuronal depolarization and release of neuropeptides such as CGRP at peripheral nerve terminals and central nerve. It is then reported that the neuropeptide enhances the sensitivity of nociceptors by binding to the relevant receptors.60 To sum up, excitatory neuropeptides such as CGRP can release from primary afferent nociceptors due to TRPV1 and consequently may play a pivotal role in initiating and developing pain.
In conclusion, as a result of this study, it was identified that NGF-TrkA signaling acts as a pivotal etiological mechanism for PTX-induced neuropathic pain. In addition, it was confirmed that the activation of the TrkA signaling pathway activates the lower signaling pathways, the Ras-MAPK and PLC-γ1 pathways. It was confirmed that TRPV1 and CGRP, whose expression is increased in PTX-induced neuropathic pain, were inhibited by TrkA antagonist treatment, indicating that the two molecules are directly affected by the activation of the TrkA signaling pathway (Figure 8). However, more direct molecular interaction experiments between AR786 and TRPV1 and CGRP are needed to confirm a direct effect.
 |
Figure 8 Schematic representation of the pathways. In nociceptive neurons, NGF plays an important role in pain and represents its initiation. Re-efflux of NGF and activation of TrkA, a tyrosine kinase that binds to NGF receptors, is induced by inflammation. NGF-binding induces TrkA dimerization, autophosphoryla tion and subsequent binding and activation of PLCγ.56,57 In this study, increased PKC expression was observed in the DRG of the CIPN mouse model. This finding provides a potential mechanism and target by which persistent activation of PKC may contribute to CIPN hyperalgesia.38 |
Our findings may therefore improve our understanding of NGF/trkA signaling mechanisms in nociceptive symptoms associated with PTX-induced neuropathic pain. In addition, the development of a targeted therapy using TrkA is expected to be presented as a new treatment for PTX-induced neuropathy.
Conclusion
Taken together, the NGF gene expression and TrkA receptor activation in the DRG of animal models after PTX treatment can be co-expressed with the NGF/TrkA downstream proteins PKC, TRPV1, and CGRP. It suggests that each protein may be involved in pain signals in PTX-induced peripheral neuropathy.
Ethics Approval and Informed Consent
All procedures in the current study were examined and approved by the Institutional Animal Care and Use Committee (CUP AEC 2023-003) of the Catholic University of Pusan and were conducted in compliance with the ethical guidelines of the National Institutes of Health and the International Association for the Study of Pain.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (Nos. NRF-2022R1F1A1074419) and the BB21plus funded by Busan Metropolitan City and Busan Techno Park.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hagiwara H, Sunada Y. Mechanism of taxane neurotoxicity. Breast Cancer. 2004;11(1):82–85. doi:10.1007/BF02968008
2. Chaudhry V, Rowinsky EK, Sartorius SE, Donehower RC, Cornblath DR. Peripheral neuropathy from taxol and cisplatin combination chemotherapy: clinical and electrophysiological studies. Ann Neurol. 1994;35(3):304–311. doi:10.1002/ana.410350310
3. Lee JJ, Swain SM. Peripheral neuropathy induced by microtubule-stabilizing agents. J Clin Oncol. 2006;24(10):1633–1642. doi:10.1200/JCO.2005.04.0543
4. Park S, Krishnan A, Lin C, Goldstein D, Friedlander M, Kiernan M. Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Curr Med Chem. 2008;15(29):3081–3094. doi:10.2174/092986708786848569
5. Sisignano M, Baron R, Scholich K, Geisslinger G. Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat Rev Neurol. 2014;10(12):694–707. doi:10.1038/nrneurol.2014.211
6. Dougherty PM, Cata JP, Cordella JV, Burton A, Weng H-R. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109(1–2):132–142. doi:10.1016/j.pain.2004.01.021
7. Wickham R. Chemotherapy-induced peripheral neuropathy: a review and implications for oncology nursing practice. Clin J Oncol Nurs. 2007;11(3):361. doi:10.1188/07.CJON.361-376
8. Chen Y, Yang C, Wang Z. Proteinase-activated receptor 2 sensitizes transient receptor potential vanilloid 1, transient receptor potential vanilloid 4, and transient receptor potential ankyrin 1 in paclitaxel-induced neuropathic pain. Neuroscience. 2011;193:440–451. doi:10.1016/j.neuroscience.2011.06.085
9. Li Y, Zhang H, Zhang H, Kosturakis AK, Jawad AB, Dougherty PM. Toll-like receptor 4 signaling contributes to Paclitaxel-induced peripheral neuropathy. J Pain. 2014;15(7):712–725. doi:10.1016/j.jpain.2014.04.001
10. Ledeboer A, Jekich BM, Sloane EM, et al. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun. 2007;21(5):686–698. doi:10.1016/j.bbi.2006.10.012
11. Luo J, Bavencoffe A, Yang P, et al. Zinc inhibits TRPV1 to alleviate chemotherapy-induced neuropathic pain. J Neurosci. 2018;38(2):474–483.
12. Sofroniew MV, Howe CL, Mobley WC. Nerve growth factors signaling, neuroprotection, and neural repair. Annual Rev Neurosci. 2001;24(1):1217. doi:10.1146/annurev.neuro.24.1.1217
13. Khan N, Smith MT. Neurotrophins and neuropathic pain: role in pathobiology. Molecules. 2015;20(6):10657–10688. doi:10.3390/molecules200610657
14. Pathak NN, Balaganur V, Lingaraju MC, et al. Antihyperalgesic and anti-inflammatory effects of atorvastatin in chronic constriction injury-induced neuropathic pain in rats. Inflammation. 2013;36(6):1468–1478. doi:10.1007/s10753-013-9688-x
15. Vivoli E, Mannelli LDC, Salvicchi A, et al. Acetyl-L-carnitine increases artemin level and prevents neurotrophic factor alterations during neuropathy. Neuroscience. 2010;167(4):1168–1174. doi:10.1016/j.neuroscience.2010.03.017
16. Fukuoka T, Kondo E, Dai Y, Hashimoto N, Noguchi K. Brain-derived neurotrophic factor increases in the uninjured dorsal root ganglion neurons in selective spinal nerve ligation model. J Neurosci. 2001;21(13):4891–4900. doi:10.1523/JNEUROSCI.21-13-04891.2001
17. Mantyh PW, Koltzenburg M, Mendell LM, Tive L, Shelton DL, Warner DS. Antagonism of nerve growth factor-TrkA signaling and the relief of pain. J Am Soc Anesthesiologists. 2011;115(1):189–204.
18. Culshaw AJ, Bevan S, Christiansen M, et al. Identification and biological characterization of 6-aryl-7-isopropylquinazolinones as novel TRPV1 antagonists that are effective in models of chronic pain. J Med Chem. 2006;49(2):471–474. doi:10.1021/jm051058x
19. Pomonis JD, Harrison JE, Mark L, Bristol DR, Valenzano KJ, Walker K. N-(4-Tertiarybutylphenyl)-4-(3-cholorphyridin-2-yl)tetrahydropyrazine −1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties: II. in vivo characterization in rat models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003;306(1):387–393. doi:10.1124/jpet.102.046268
20. Cui M, Honore P, Zhong C, et al. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J Neurosci. 2006;26(37):9385–9393. doi:10.1523/jneurosci.1246-06.2006
21. Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov. 2007;6(5):357–372. doi:10.1038/nrd2280
22. Moran MM, McAlexander MA, Bíró T, Szallasi A. Transient receptor potential channels as therapeutic targets. Nat Rev Drug Discov. 2011;10(8):601–620. doi:10.1038/nrd3456
23. Kalynovska N, Adamek P, Palecek J. TRPV1 receptors contribute to paclitaxel-induced c-Fos expression in spinal cord dorsal horn neurons. Physiological Res. 2017;66(3):549. doi:10.33549/physiolres.933613
24. McGaraughty S, Chu KL, Brown BS, et al. Contributions of central and peripheral TRPV1 receptors to mechanically evoked and spontaneous firing of spinal neurons in inflamed rats. J Neurophysiol. 2008;100(6):3158–3166. doi:10.1152/jn.90768.2008
25. Holzer P. The pharmacological challenge to tame the transient receptor potential vanilloid-1 (TRPV1) nocisensor. Br J Pharmacol. 2008;155(8):1145–1162. doi:10.1038/bjp.2008.351
26. Christoph T, Grünweller A, Mika J, et al. Silencing of vanilloid receptor TRPV1 by RNAi reduces neuropathic and visceral pain in vivo. Biochem Biophys Res Commun. 2006;350(1):238–243. doi:10.1016/j.bbrc.2006.09.037
27. Priestley JV, Michael GJ, Averill S, Liu M, Willmott N. Regulation of nociceptive neurons by nerve growth factor and glial cell line derived neurotrophic factor. Can J Physiol Pharmacol. 2002;80(5):495–505. doi:10.1139/y02-034
28. Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51(2):159–212.
29. Krukowski K, Nijboer CH, Huo X, Kavelaars A, Heijnen CJ. Prevention of chemotherapy-induced peripheral neuropathy by the small-molecule inhibitor pifithrin-μ. Pain. 2015;156(11):2184. doi:10.1097/j.pain.0000000000000290
30. Mao-Ying Q-L, Kavelaars A, Krukowski K, et al. The anti-diabetic drug metformin protects against chemotherapy-induced peripheral neuropathy in a mouse model. PLoS One. 2014;9(6):e100701. doi:10.1371/journal.pone.0100701
31. Singhmar P, Huo X, Li Y, et al. An orally active Epac inhibitor reverses mechanical allodynia and loss of intraepidermal nerve fibers in a mouse model of chemotherapy-induced peripheral neuropathy. Pain. 2018;159(5):884. doi:10.1097/j.pain.0000000000001160
32. Falah M, Rayan M, Rayan A. A Novel Paclitaxel Conjugate with Higher Efficiency and Lower Toxicity: a New Drug Candidate for Cancer Treatment. Int J Mol Sci. 2019;20(19):4965. doi:10.3390/ijms20194965
33. Ashraf S, Bouhana KS, Pheneger J, Andrews SW, Walsh DA. Selective inhibition of tropomyosin-receptor-kinase A (TrkA) reduces pain and joint damage in two rat models of inflammatory arthritis. Arthritis Res Therapy. 2016;18(1):1–11. doi:10.1186/s13075-016-0996-z
34. Nwosu LN, Mapp PI, Chapman V, Walsh DA. Blocking the tropomyosin receptor kinase A (TrkA) receptor inhibits pain behaviour in two rat models of osteoarthritis. Ann Rheumatic Dis. 2016;75(6):1246–1254. doi:10.1136/annrheumdis-2014-207203
35. Lee S, Hwang C, Marini S, et al. NGF-TrkA signaling dictates neural ingrowth and aberrant osteochondral differentiation after soft tissue trauma. Nat Commun. 2021;12(1):4939. doi:10.1038/s41467-021-25143-z
36. Masocha W, Parvathy SS. Preventative and therapeutic effects of a GABA transporter 1 inhibitor administered systemically in a mouse model of paclitaxel-induced neuropathic pain. PeerJ. 2016;4:e2798. doi:10.7717/peerj.2798
37. Schulz A, Walther C, Morrison H, Bauer R. In vivo electrophysiological measurements on mouse sciatic nerves. JoVE. 2014;2014(86):e51181.
38. Sikand P, Premkumar LS. Potentiation of glutamatergic synaptic transmission by protein kinase C-mediated sensitization of TRPV1 at the first sensory synapse. J Physiol. 2007;581(Pt 2):631–647. doi:10.1113/jphysiol.2006.118620
39. Chuang -H-H, Prescott ED, Kong H, et al. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411(6840):957–962. doi:10.1038/35082088
40. Pace A, Nisticò C, Cuppone F, et al. Peripheral neurotoxicity of weekly paclitaxel chemotherapy: a schedule or a dose issue? Clin Breast Cancer. 2007;7(7):550–554. doi:10.3816/CBC.2007.n.010
41. Spicarova D, Palecek J. The role of the TRPV1 endogenous agonist N-Oleoyldopamine in modulation of nociceptive signaling at the spinal cord level. J Neurophysiol. 2009;102(1):234–243. doi:10.1152/jn.00024.2009
42. Ta LE, Bieber AJ, Carlton SM, Loprinzi CL, Low PA, Windebank AJ. Transient Receptor Potential Vanilloid 1 is essential for cisplatin-induced heat hyperalgesia in mice. Mol Pain. 2010;6:15. doi:10.1186/1744-8069-6-15
43. Jimenez-Andrade JM, Peters CM, Mejia NA, Ghilardi JR, Kuskowski MA, Mantyh PW. Sensory neurons and their supporting cells located in the trigeminal, thoracic and lumbar ganglia differentially express markers of injury following intravenous administration of paclitaxel in the rat. Neurosci Lett. 2006;405(1–2):62–67. doi:10.1016/j.neulet.2006.06.043
44. Obata K, Noguchi K. MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci. 2004;74(21):2643–2653. doi:10.1016/j.lfs.2004.01.007
45. Ji -R-R, Gereau IVRW, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60(1):135–148. doi:10.1016/j.brainresrev.2008.12.011
46. Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain®. 2014;155(12):2461–2470. doi:10.1016/j.pain.2014.09.020
47. Kim HK, Hwang SH, Abdi S. Tempol Ameliorates and Prevents Mechanical Hyperalgesia in a Rat Model of Chemotherapy-Induced Neuropathic Pain. Front Pharmacol. 2016;7:532. doi:10.3389/fphar.2016.00532
48. Nishida T, Tsubota M, Kawaishi Y, et al. Involvement of high mobility group box 1 in the development and maintenance of chemotherapy-induced peripheral neuropathy in rats. Toxicology. 2016;365:48–58. doi:10.1016/j.tox.2016.07.016
49. Matsumoto M, Inoue M, Hald A, Xie W, Ueda H. Inhibition of paclitaxel-induced A-fiber hypersensitization by gabapentin. J Pharmacol Exp Ther. 2006;318(2):735–740. doi:10.1124/jpet.106.103614
50. Segat GC, Manjavachi MN, Matias DO, et al. Antiallodynic effect of β-caryophyllene on paclitaxel-induced peripheral neuropathy in mice. Neuropharmacology. 2017;125:207–219. doi:10.1016/j.neuropharm.2017.07.015
51. Tofthagen C, McAllister RD, Visovsky C. Peripheral neuropathy caused by Paclitaxel and docetaxel: an evaluation and comparison of symptoms. J Adv Practitioner Oncol. 2013;4(4):204.
52. Wieseler-Frank J, Maier SF, Watkins LR. Immune-to-brain communication dynamically modulates pain: physiological and pathological consequences. Brain Behav Immun. 2005;19(2):104–111. doi:10.1016/j.bbi.2004.08.004
53. Binshtok AM, Wang H, Zimmermann K, et al. Nociceptors are interleukin-1β sensors. J Neurosci. 2008;28(52):14062–14073. doi:10.1523/JNEUROSCI.3795-08.2008
54. Peters CM, Jimenez-Andrade JM, Kuskowski MA, Ghilardi JR, Mantyh PW. An evolving cellular pathology occurs in dorsal root ganglia, peripheral nerve and spinal cord following intravenous administration of paclitaxel in the rat. Brain Res. 2007;1168:46–59. doi:10.1016/j.brainres.2007.06.066
55. Drexhage RC, van der Heul-Nieuwenhuijsen L, Padmos RC, et al. Inflammatory gene expression in monocytes of patients with schizophrenia: overlap and difference with bipolar disorder. A study in naturalistically treated patients. International Journal of Neuropsychopharmacology. 2010;13(10):1369–1381. doi:10.1017/S1461145710000799
56. Obermeier A, Lammers R, Wiesmüller K, Jung G, Schlessinger J, Ullrich A. Identification of Trk binding sites for SHC and phosphatidylinositol 3′-kinase and formation of a multimeric signaling complex. J Biol Chem. 1993;268(31):22963–22966. doi:10.1016/S0021-9258(19)49410-6
57. Pena DA, Duarte ML, Pramio DT, Devi LA, Schechtman D. Exploring morphine-triggered PKC-targets and their interaction with signaling pathways leading to pain via TrkA. Proteomes. 2018;6(4):39. doi:10.3390/proteomes6040039
58. Li Y, Adamek P, Zhang H, et al. The cancer chemotherapeutic paclitaxel increases human and rodent sensory neuron responses to TRPV1 by activation of TLR4. J Neurosci. 2015;35(39):13487–13500. doi:10.1523/JNEUROSCI.1956-15.2015
59. Nakahashi Y, Kamiya Y, Funakoshi K, et al. Role of nerve growth factor-tyrosine kinase receptor A signaling in paclitaxel-induced peripheral neuropathy in rats. Biochem Biophys Res Commun. 2014;444(3):415–419. doi:10.1016/j.bbrc.2014.01.082
60. Quartu M, Carozzi VA, Dorsey S, et al. Bortezomib treatment produces nocifensive behavior and changes in the expression of TRPV1, CGRP, and substance P in the rat DRG, spinal cord, and sciatic nerve. Biomed Res. Int. 2014;2014:1–19. doi:10.1155/2014/180428
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.