Back to Journals » Clinical Ophthalmology » Volume 19
Utilizing Corneal Fluorescein and Conjunctival Lissamine Green Staining Combined with in vivo Confocal Microscopy for Grading the Severity of Dry Eye Disease
Authors Guo M, Huang B, Jia X, Liu B
Received 1 October 2024
Accepted for publication 12 February 2025
Published 10 March 2025 Volume 2025:19 Pages 807—817
DOI https://doi.org/10.2147/OPTH.S498893
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mian Guo,1 Bo Huang,2 Xiaokang Jia,3 Bo Liu2
1Department of Ophthalmology, Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, 56300, People’s Republic of China; 2Key Laboratory of Basic Pharmacology of Ministry of Education, Jointly Constructed by Province and Ministry, Zunyi Medical University, Zunyi, Guizhou, 563000, People’s Republic of China; 3School of Traditional Chinese Medicine, Hainan Medical University, Haikou, Hainan, 571199, People’s Republic of China
Correspondence: Bo Liu, Email [email protected]; [email protected]
Objective: To explore the application of corneal fluorescein sodium (CFS)-conjunctival lissamine green (CLG) staining combined with in vivo confocal microscopy (IVCM) in clinical grading of the severity of dry eye disease (DED).
Methods: Eleven normal persons (4 males and 7 females) and thirty-two mild, moderate, and severe DED patients (aged 22 to 56 years, and mean age 45.4 ± 12.9 years;14 males and 18 females) were included for CFS-CLG staining. The stained positive areas were observed and recorded for the number and morphology of staining points. Subsequently, the morphology and cell density of corneal and conjunctival cells were observed and analyzed by IVCM.
Results: CFS-CLG staining revealed that the number of CFS-stained points were not significantly increased in mild DED, and there was a small amount of CLG staining in the temporal bulbar conjunctiva; the CFS-stained points of moderate DED were increased compared with mild DED, and the nasal conjunctiva diffuse small flake CLG staining was observed; the cornea of severe DED had the most fluorescein-stained points, and the conjunctiva diffuse large CLG staining. IVCM examination showed that corneal epithelial basal cell density was significantly decreased, while activated corneal Langerhans cells were significantly increased in moderate and severe DED. Meanwhile, the morphology of superficial stromal cells of cornea became irregular and the cell density decreased significantly in moderate and severe DED. The density of conjunctival goblet cells was also significantly reduced in moderate and severe DED. Moreover, the density of activated conjunctival Langerhans cells increased significantly in mild, moderate, and severe DED.
Conclusion: Observing and detecting the morphology and density of corneal epithelial basal cells, superficial stromal cells, corneal Langerhans cells, conjunctival goblet cells, and conjunctival Langerhans cells in positive CFS-CLG stained areas by IVCM, which may be a reliable basis for clinical grading of DED severity.
Keywords: dry eye disease, corneal fluorescein sodium-conjunctival lissamine green staining, in vivo confocal microscopy
Graphical Abstract:

Introduction
Dry eye disease (DED) is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.1,2 The global incidence of DED is between 5% and 50%, while in China, the incidence of DED is about 21% to 30%.3 People with DED usually have symptoms such as dryness, foreign body sensation, photophobia, blurred or fluctuating vision, which can seriously affect work and daily life.4 Traditionally, DED was believed to primarily affect adults over 40 years old, but in recent years, possibly influenced by the widespread use of electronic devices and environmental factors, the prevalence of DED has been increasing annually and trending toward younger populations.5
The Tear Film & Ocular Surface Society (TFOS) organization,6 also Chinese expert provide a detailed explanation of DED diagnostic criteria for DED mainly based on the combination of symptoms, signs, tear breakup time, corneal fluorescein sodium staining, and tear secretion tests.7,8 Also diagnosed with questionnaire based methods.9 However, these methods are limited by the examination conditions, such as the light of slit-lamp, the amount of fluorescein dripping into the eyes, and the stimulation of test paper caused the increase of tear secretion, which affected the test results, and the specificity was not high.10 Although conjunctival impression cytology is specific in the diagnosis of early DED, it has limitations in clinical practice.11 Therefore, there is an urgent need for a rapid, reliable and accurate method to diagnose DED and its severity.
DED usually involves epithelial changes in the cornea and conjunctiva, which makes corneal and conjunctiva staining essential for intuitive and convenient assessment of inflammation and epithelial cell damage, and is an important criterion for grading the severity of DED.12 However, there is a regrettable lack of support from histopathology. Fortunately, the emergence of in vivo confocal microscopy (IVCM) significantly reduced the number of pathological biopsies, more accurately defined the site and nature of the lesion, and improved the diagnostic efficiency.13 The TFOS organization proposes IVCM as an emerging technology that appears to have several potential applications in research and clinical practice and may prove to be a good candidate to develop and to validate predictive biomarkers and surrogate endpoints for clinical research on DED.6
IVCM (Heidelberg Retina Tomograph III, Heidelberg Engineering, Germany) enables non-invasive, high-resolution, real-time dynamic detection and recording of corneal and conjunctival structures at the cellular level.14 The Rostock Cornea Module allows rapid, reliable, and accurate acquisition and analysis of microstructures across various layers of the cornea and conjunctiva. IVCM also enables observation and quantitative examination of langerhans cells in vivo.15 Many studies have shown that IVCM is an emerging, non-invasive technique for assessing ocular surface diseases at the living cell level.1 In the field of DED, IVCM can observe the morphology and density of cells in each layer of cornea and conjunctiva non-invasively, as well as the morphology and density of nerve fibers, goblet cells and inflammatory cells, which is a powerful method for evaluating DED.16 In 2020, the China dry eye expert consensus group launched a new version of the DED grading scheme based on the principle of focusing on clinical practice. Depending on the severity of the signs, DED can be classified into mild, moderate, and severe levels. The precise grading of DED can help clinical doctors choose the appropriate treatment.17 However, the clinical application of IVCM in the classification of DED severity has not been published. In this study, IVCM will be used to observe and calculate the changes in the morphology and density of corneal and conjunctiva cells in each layer of the staining positive area, so as to provide the basic experimental basis for clinical classification of the severity of DED by IVCM.
Methods
General Information
Eleven normal persons (4 males and 7 females), and a total of 32 DED patients who visited the Department of Ophthalmology of the Second Affiliated Hospital of Zhejiang University School from May 2020 to July 2020 were enrolled in this study. Among them were 14 males and 18 females, aged 22 to 56 years (mean age 45.4 ± 12.9 years). The disease duration ranged from 6 to 38 weeks, with an average of 20.49 ± 9.65 weeks. The study was approved by the Medical Ethics Committee of Affiliated Hospital of Zunyi Medical University (Ethics Approval No. 2020–12). We conducted this study in accordance with the Tenets of the World Medical Association’s Declaration of Helsinki.
Inclusion and Exclusion Criteria
Inclusion Criteria
Patients with clinically diagnosed as DED by the clinical ophthalmology department of Affiliated Hospital of Zunyi Medical University. The criteria of DED classification according to Chinese expert consensus on dry eye.7 If the condition is the same in both eyes, the right eye will be chosen for observation, and the eye with the worse condition was selected for observation when the condition of both eyes was different. During the clinical research, DED patients have a high compliance with the research project, and they are familiar with the content of this study and sign the informed consent. In order to minimize bias and unconscious suggestion, the doctors involved in the diagnosis of dry eye grade did not participate in the subsequent experiment.
Exclusion Criteria
① History of allergy to the components used in this study. ② Visual fatigue or dryness caused by other eye diseases. ③ Patients with insufficient liver and kidney function. ④ Patients who had undergone bone marrow transplantation. ⑤ Patients with cognitive and psychiatric disorders.
Corneal Fluorescein Staining and Conjunctival Lissamine Green Staining
Corneal Fluorescein Staining
Utilized 0.5% ofloxacin eye drops (produced by Shandong Xinhua Pharmaceutical Co., Ltd., H20113438) to moisten the lissamine green or fluorescein sodium test paper (produced by Tianjin Jingming New Technology Development Co., Ltd., 20100040). The test paper was placed in contact with the lower conjunctival fornix, and patients blinked 3–4 times to spread fluorescein onto the ocular surface. Observations and photography were conducted under a slit lamp biomicroscope (Chongqing Kanghuarui Ming Technology Co., Ltd., SLM-2ER) with cobalt blue light to observe the stained area.18
Conjunctival Lissamine Green Staining
Utilized 0.5% ofloxacin eye drops (produced by Shandong Xinhua Pharmaceutical Co., Ltd., H20113438) to moisten the lissamine green or fluorescein sodium test paper (produced by Tianjin Jingming New Technology Development Co., Ltd., 20100040). The test paper was placed in contact with the lower eyelid margin, and patients blinked 3–4 times to spread lissamine green onto the ocular surface. After closing their eyes for 1–4 minutes, observations were made under white light with a slit lamp biomicroscope (Chongqing Kanghuarui Ming Technology Co., Ltd., SLM-2ER) to observe the location of conjunctival staining and capture photographs.19
In vivo Confocal Microscopy (IVCM) Observation
IVCM (Heidelberg engineering, Germany, Rostock Cornea Module) was used to observe the stained positive areas of the cornea and conjunctiva. The laser wavelength was 670 nm, the field of view was 400 μm × 400 μm, with a magnification of 800 times, and a resolution of 1 μm.
In order to minimize bias and unconscious suggestion, the experimenter who performed corneal fluorescein sodium-conjunctival lissamine green staining and the experimenters who performed IVCM were different, and each examiner being blind to the other’s results. The patient underwent topical anesthesia with 0.5% promecaine hydrochloride (s.a. Alcon-Couvreur n.v., H20090082). Apply 0.2% carpom eye gel (Shandong Bausch & Lomb Freda Pharmaceutical Co. Ltd, H20090924) on the surface of the objective lens, then cover the objective lens with a sterile corneal contact cap, and apply another drop of 0.2% carpom eye gel on the outside of the sterile corneal contact cap. Participants’ head was fixated on a forehead and chin rest, gaze position of the dominant eye was tracked with an eye-tracker.
The lens position was adjusted to allow the corneal contact cap to micro-contact the cornea, with the preset focal plane depth at 0 μm. The focal plane adjustment ring was rotated to obtain images of different depth and different levels of the cornea. Pay special attention to examine areas of conjunctiva that are positive for lissamine green staining. The head position and lens fit were adjusted according to the position to obtain clear images. Valuable clear images were selected and saved using a monitor. All procedures were performed by the same technician.
Using the in vivo laser confocal microscopy’s built-in cell counting system, the density of corneal epithelial basal cells, conjunctival epithelial cells, conjunctival basal cells, Langerhans cells and goblet were calculated. The cell density will be calculated as: total cell count/ 0.16 mm2 (PCS/mm2).20
Statistical Analysis
The data were analyzed by SPSS 16.0 statistics software and values were expressed as mean ± SD. Normally distributed variables were assessed using one-way ANOVA, and followed by Bonferroni test, and not normally distributed were followed by Dunnett’s T3. P < 0.05 was considered statistically significant.
Results
Corneal Fluorescein Staining and Conjunctival Lissamine Green Staining
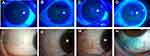
According to the previous standards,21 there were 11 patients with mild DED, 9 patients with moderate DED, and 12 patients with severe DED in this study. As shown in the Figure 1, compared with normal people, patients with mild DED had the fewest corneal fluorescein staining spots (less than 5, Figure 1B), and a small number of punctate lissamine green staining in the temporal bulbar conjunctiva (Figure 1ii). Corneal fluorescent staining points increased in patients with moderate DED compared with those with mild DED (5–30 spots, Figure 1C), and the nasal bulbar conjunctiva has diffused small patches of green staining (Figure 1iii). Patients with severe DED had the most corneal fluorescein staining spots (more than 30), and there were small fusion patches on the nasal side and below (Figure 1D). Moreover, the bulbar conjunctiva diffuse large conjunctival lissamine green staining (Figure 1iv).
Corneal Basal Epithelial Cells
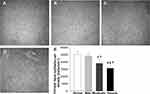
As shown in the Figure 2, compared with normal people, examination with IVCM revealed that in patients with mild DED, the morphology of corneal basal epithelial cells was generally normal, with densely packed polygonal cells exhibiting alternated light and dark areas. Cell borders were highly reflective, cytoplasm was gray or had low reflectivity, and cell count was normal (Figure 2B). In patients with moderate DED, some corneal epithelial basal cells had unclear borders (Figure 2C), and compared to mild DED patients, cell density was significantly reduced (Figure 2E, p < 0.05). Compared to patients with mild and moderate DED, those with severe DED exhibited unclear cell borders, irregular cell morphology, and disordered arrangement of cells (Figure 2D), with a significant decrease in cell density (Figure 2E, p < 0.05).
Corneal Langerhans Cells
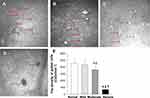
In normal corneas, few or no activated Langerhans cells (LCs) are typically observed (Figure 3). IVCM examination revealed that in mild DED patients (Figure 3A), there were few mildly activated LCs in the subepithelial nerve plexus of the cornea (Figure 3B). In patients with moderate DED, highly reflective activated LCs were found in the corneal subepithelial plexus, which were morphologically diverse and interwoven into a web (Figure 3C). In patients with severe DED, activated LCs were dendritic interwoven into a network, with longer and more pronounced dendrites compared to moderate DED patients, and there was a significant increase in LCs density (Figure 3D and E, p < 0.05).
Superficial Corneal Stromal Cells
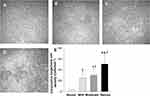
Examination with IVCM revealed that in patients with mild DED (Figure 4A), the density of superficial corneal stromal cells was high, the cell nucleus was elliptical and highly reflective, and the cell body was weakly reflective (Figure 4B). In patients with moderate DED, the activation of some shallow superficial corneal stromal cells was polygonal and stretched horizontally and horizontally (Figure 4C), and the cell density was significantly lower than that in patients with mild DED (Figure 4E, p < 0.05). In patients with severe DED, the density of the superficial corneal stromal cells was decreased (Figure 4E, p < 0.05), the nuclear morphology was irregular, and some of the superficial corneal stromal cells were activated in polygonal shape and stretched out in a network (Figure 4D).
Conjunctival Goblet Cells
Normal conjunctival goblet cells are 2 to 3 times larger than the surrounding conjunctival epithelium cells, and the cytoplasm is filled with translucent granules and sometimes with reflective nuclei (Figure 5A). IVCM examination revealed that in patients with mild DED, goblet cells were normal in size, the cytoplasm was bright, a circular low reflection band was seen around the cells, the nucleus was occasionally visible, the nuclear reflection was low, and the goblet cells showed small bright spots (Figure 5B). In patients with moderate DED, the density of conjunctival goblet cells was decreased, and the brightness and volume of the cells were also slightly decreased (Figure 5C). Moreover, the density of conjunctival goblet cells is further reduced in severe dry eye patients, and conjunctival microcysts appear, which represents the state of conjunctival goblet cell failure (Figure 5D).
Conjunctival Langerhans Cells
IVCM examination showed that compared with normal people (Figure 6A), patients with mild DED had a small amount of conjunctival LCs dispersion (Figure 6B). Conjunctival LCs from patients with moderate DED showed dendritic protrusions, marked activation, and hyperreflective granules in the central nucleus (Figure 6C). The number of LCs increased significantly in patients with severe DED (p < 0.05), and the activated LCs were more dense and dendrites were more obvious (Figure 6D, p < 0.05).
Discussion
Consistent with previous studies, the number of corneal fluorescent staining spots and the area of conjunctival lissamine green staining were significantly different in patients with different severity of DED.7 Furthermore, our study has found that through IVCM observation of areas positive for corneal fluorescein-conjunctival lissamine green staining, significant differences were noted in the morphology and density of corneal basal epithelial cells, superficial corneal stromal cells, and conjunctival goblet cells among mild, moderate, and severe DED patients. The density of corneal and conjunctival LCs also increased with the severity of DED.
DED particularly damages the cornea, which can cause dry spots on the cornea, secondary filamentous keratitis, and even corneal ulcer and perforation.22 Corneal fluorescein-conjunctival lissamine green staining is an important method for diagnosing and evaluating the severity of dry eye. Corneal fluorescein-conjunctival lissamine green staining can clearly understand the location and extent of corneal epithelial defects and conjunctival epithelium not covered by mucin.23 Consistent with previous studies,12,24 as the severity of dry eye increased, the corneal fluorescent staining points gradually increased, and the conjunctival Lissamine green staining changed from a few dots to a diffuse large flake in this study.
DED can lead to delayed healing or non-healing after corneal conjunctival epithelial injury. Corneal basal epithelial cells are columnar cells at the base of corneal epithelial cells, which play an important bridging role in corneal epithelial renewal and post-injury repair.25 Previous observations from the central cornea by IVCM showed that the density of corneal basal epithelial cells decreased gradually with the severity of dry eye.26 Our study also observed a significant decrease in the density of corneal basal epithelial cells in mild, moderate, and severe DED patients through IVCM (P<0.05).
The stromal layer of the cornea is the main component of the cornea, accounting for 90% of the corneal thickness. As we know, the stromal layer of the cornea is composed of corneal stromal cells, collagen fibers and extracellular matrix. Corneal stromal layer has the characteristics of regular structure and high transparency. Under normal circumstances, corneal stromal cells secrete components of the stromal layer to maintain the transparency of the cornea. Therefore, corneal stromal cells are of great significance for the repair of corneal injury. Under normal circumstances, corneal stromal cells are in a quiescent state. However, when corneal injury occurs, different epithelial-derived factors and environmental signals will affect the activity of corneal stromal cells and the corneal repair function. However, the superficial corneal stromal cells of DED patients are highly reflective, which is due to the activation of stromal cells.27
Normally, corneal stromal cells are long spindle-shaped, with small volume of cell body and nucleus, strong reflection in nucleus and weak reflection in cytoplasm. As the severity of dry eye increased, the density of superficial corneal stromal cells of patients with mild dry eye gradually decreased. Consistent with previous studies, the corneal stromal cells of mild dry eye patients showed a polygonal activation state with protrusion, with larger volume of cell body and nucleus, stronger reflection in nucleus, weaker reflection in cytoplasm, and unclear boundary between nucleus and cytoplasm.28 In patients with moderate DED, the activation of superficial corneal stromal cells increased. In patients with severe DED, the cells showed a “crab-like” activation state, which may be related to the severe apoptosis of superficial corneal stromal cells in severe DED patients, and the impairment of epithelial barrier function led to inflammation affecting the superficial corneal stroma.
The core pathological mechanism of DED is increased osmotic pressure of tears and chronic inflammatory response of ocular surface. Corneal LCs are professional antigen presenting cells in the immune system of ocular surface and participate in chronic inflammatory response of DED. The functional status of corneal LCs has a profound influence on the occurrence, development and outcome of DED. Previous studies have shown that the severity of DED is positively correlated with the density of LCs.9,10 In this study, the number of LCs domain dendrites increased with the increase of the severity of DED in the positive region stained by corneal fluorescein-conjunctival lissamine green staining.
Conjunctival goblet cells are scattered among conjunctival epithelial cells and are an important part of the conjunctival epithelium. They can secrete mucin to protect and lubricate the ocular surface and other bioactive substances.29 Previous studies have shown that DED can cause changes in conjunctival goblet cell density.11 This study also found that the density of conjunctival goblet cells in positive corneal fluorescein-conjunctival lissamine green staining decreased significantly with the increase of DED severity, and the brightness and volume decreased with the increase of DED severity, and conjunctival goblet cell failure even occurred in patients with severe DED.
In recent years, goblet cells have been found to participate in antigen presentation, regulate the differentiation of dendritic cell phenotypes, induce immune tolerance, and participate in the occurrence and development of DED.11 This study also found that DED not only caused the change of goblet cells, but also caused the activation of conjunctiva LCs in the positive region of corneal fluorescein-conjunctival lissamine green staining, and the number of conjunctiva LCs increased significantly with the increase in the severity of DED.
Above all, we believe the advantages of IVCM in the diagnosis of DED are that that the cells, inflammatory factors, and the number, shape and distribution of nerves in all layers of the ocular surface (eyelid, cornea, conjunctiva, etc.) can be observed from the cellular level in a non-invasive manner, so that the damage of DED to the ocular surface can be more accurately and objectively reflected, which is conducive to accurately guiding the treatment of DED. However, the disadvantage of IVCM is that the examination time is longer, the children’s cooperation is poor, and a higher level of ophthalmic inspectors is required. Meanwhile, IVCM is not accessible to everyone.30
Conclusion
In vivo non-invasive observation and detection the morphology and quantity of corneal basal epithelial cells, superficial corneal stromal cells, conjunctival goblet cells, and LCs through IVCM in positive areas of corneal fluorescein-conjunctival lissamine green staining can serve as reliable evidence for clinically diagnosing and grading DED severity.
Acknowledgment
The present study was supported by Guizhou Science and Technology Department ZK (2023-575,2021-428); Zunyi City Science and Technology Cooperation HZ (2022-245); Guizhou Science and Technology Department [No. QIANKE HEZHICHEN (2023) YIBAN576]; Zunyi Science and Technology Department [Serial No. HZ359 2022)]; Science and Technology Plan Project of Zunyi City [No. Zunshi Kehe Support GY(2021)65].
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Chang YW, Sun YL, Chu E, et al.. Streptococcus thermophilus iHA318 improves dry eye symptoms by mitigating ocular surface damage in a mouse model. Microorganisms. 2024;12(7). doi:10.3390/microorganisms12071306
2. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocular Surf. 2017;15(3):276–283. doi:doi:10.1016/j.jtos.2017.05.008
3. Liu R, Zhao Y, Wu Y, et al. Individualized Chinese medicine for the treatment of diabetic patients with dry eye disease: a single-case randomized controlled protocol. Medicine. 2020;99(1):e18459. doi:10.1097/md.0000000000018459
4. Cheng E, Han K, Chen Y, et al. Associations of severity of dry eye symptoms and signs with the quality of life in the dry eye assessment and management (DREAM) study. Res Square. 2024. doi:10.21203/rs.3.rs-4738536/v1
5. Gu Q, Zheng Q, Zhang X, et al. Trends in health service use for dry eye disease from 2017 to 2021: a real-world analysis of 369,755 outpatient visits. Trans Vision Sci Technol. 2024;13(1):17. doi:10.1167/tvst.13.1.17
6. Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocular Surf. 2017;15(3):539–574. doi:10.1016/j.jtos.2017.05.001
7. Chinese expert consensus on the diagnosis and treatment of dry eye (2024)]. [Zhonghua yan ke za zhi. Chin J Ophthalmol. 2024;60(12):968–976. doi:10.3760/cma.j.cn112142-20240517-00227
8. Zou X, Nagino K, Okumura Y, et al. Optimal cutoff value of the dry eye-related quality-of-life score for diagnosing dry eye disease. Sci Rep. 2024;14(1):4623. doi:10.1038/s41598-024-55358-1
9. Patwardhan SD, Sangle A, Patwardhan NS, et al. Quantitative and qualitative assessment of evaporative dry eye post intense pulsed light therapy, a prospective study. Indian J Ophthalmol. 2024. doi:10.4103/ijo.ijo_1136_24
10. Suh A, Ong J, Waisberg E, et al. Neurostimulation as a technology countermeasure for dry eye syndrome in astronauts. Life Sci. Space Res. 2024;42:37–39. doi:10.1016/j.lssr.2024.04.003
11. Liu Y, Duan Z, Yuan J, Xiao P. Imaging assessment of conjunctival goblet cells in dry eye disease. Clin amp; Experimental Ophthalmology. 2024;52(5):576–588. doi:10.1111/ceo.14379
12. Begley CG, Caffery B, Nelson JD, et al. The effect of time on grading corneal fluorescein and conjunctival lissamine green staining. Ocular Surf. 2022;25:65–70. doi:10.1016/j.jtos.2022.05.003
13. Bucsan R, Coroleucă R, Garhöfer G, et al.. Confocal microscopy of the cornea in aqueous-deficient dry eye disease-a literature review. 2024;14(15). doi:10.3390/diagnostics14151613
14. Ibrahim OM, Matsumoto Y, Dogru M, et al. In vivo confocal microscopy evaluation of meibomian gland dysfunction in atopic-keratoconjunctivitis patients. Ophthalmology. 2012;119(10):1961–1968. doi:10.1016/j.ophtha.2012.04.001
15. Vagge A, Bonino M, Rolando M, et al. The utility of an artificial substitute to improve corneal sensitivity in glaucomatous patients on chronic therapy with prostaglandin analogs. J Ocul Pharmacol Ther. 2015;31(5):286–290. doi:10.1089/jop.2014.0131
16. Matsumoto Y, Ibrahim OMA. Application of in vivo confocal microscopy in dry eye disease. Invest Ophthalmol Visual Sci. 2018;59(14):Des41–des47. doi:10.1167/iovs.17-23602
17. Patel C, Supramaniam D. When the eyes are dry: an algorithm approach and management in general practice. Aust J Gen Pract. 2021;50(6):369–376. doi:10.31128/ajgp-04-20-5318
18. Yin J, Wu Z. Sodium hyaluronate and pranoprofen improve visual function and reduce inflammation in patients with dry eye. Immuno Immunotoxicology. 2024;1–8. doi:10.1080/08923973.2024.2390449
19. Zhao L, Duan H, Ma B, et al. Impact of topical 0.05% cyclosporine a eye drops on post-femtosecond-assisted laser in situ keratomileusis ocular surface recovery: a randomized clinical trial. Eye Contact Lens. 2024;50(8):348–356. doi:10.1097/icl.0000000000001103
20. Shareef O, Soleimani M, Tu E, et al. A novel artificial intelligence model for diagnosing Acanthamoeba keratitis through confocal microscopy. Ocular Surf. 2024;34:159–164. doi:10.1016/j.jtos.2024.07.010
21. Liu ZG. Paying attention to the expert consensus on dry eye to standardize and promote the clinical diagnosis and treatment of dry eye. [Zhonghua yan ke za zhi] Chin j Ophthalmol. 2020;56(10):726–729. doi:10.3760/cma.j.cn112142-20200714-00476
22. Zhang X, Vj M, Qu Y, et al. Dry Eye management: targeting the ocular surface microenvironment. Int J mol Sci. 2017;18(7). doi:10.3390/ijms18071398
23. Bron AJ, Argüeso P, Irkec M, et al. Clinical staining of the ocular surface: mechanisms and interpretations. Prog Retinal Eye Res. 2015;44:36–61. doi:10.1016/j.preteyeres.2014.10.001
24. Begley C, Caffery B, Chalmers R, et al. Review and analysis of grading scales for ocular surface staining. Ocular Surf. 2019;17(2):208–220. doi:10.1016/j.jtos.2019.01.004
25. Baratta RO, Del Buono BJ, Schlumpf E, et al. Collagen mimetic peptides promote corneal epithelial cell regeneration. Front Pharmacol. 2021;12(705623). doi:10.3389/fphar.2021.705623
26. Kasikci M, Erogul O, Polat O. Evaluation of aqueous-deficient and evaporative dry eye cases with confocal microscopy. J Fr Ophtalmol. 2023;46(10):1161–1168. doi:10.1016/j.jfo.2023.05.024
27. Villani E, Mantelli F, Nucci P. In-vivo confocal microscopy of the ocular surface: ocular allergy and dry eye. Curr Opin Allergy Clin Immunol. 2013;13(5):569–576. doi:10.1097/ACI.0b013e328364ec92
28. Matsumoto Y, Ibrahim OMA. Corneal in vivo laser-scanning confocal microscopy findings in dry eye patients with Sjogren's syndrome. Diagnostics 2020;10(7). doi:10.3390/diagnostics10070497
29. Cloots E, Guilbert P. Activation of goblet-cell stress sensor IRE1β is controlled by the mucin chaperone AGR2. EMBO J 2024;43(5):695–718. doi:10.1038/s44318-023-00015-y
30. Ifrah R, Quevedo L, Gantz L. Repeatability and reproducibility of Cobra HD fundus camera meibography in young adults with and without symptoms of dry eye. Ophthalmic amp; Physiological Optics. 2023;43(2):183–194. doi:10.1111/opo.13074
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.