Back to Journals » Journal of Inflammation Research » Volume 17
Vascular Endothelial Growth Factor a Promotes Chronic Itch via VEGFA-VEGFR2-PI3K-TRPV1 Axis in Allergic Contact Dermatitis
Authors Liu QY, Liu HF, Ye LQ, Li T , Chen ZM, Wang Y, Peng Z, Wan L
Received 23 May 2024
Accepted for publication 27 September 2024
Published 17 October 2024 Volume 2024:17 Pages 7423—7439
DOI https://doi.org/10.2147/JIR.S470094
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Qin-Yu Liu,1,2 Hua-Feng Liu,1,2 Liu-Qing Ye,1,2 Tian Li,1,2 Zuo-Ming Chen,1,2 Yu Wang,1,2 Zhe Peng,1,2 Li Wan1
1Department of Pain Medicine, The State Key Clinical Specialty in Pain Medicine, The Second Affiliated Hospital, Guangzhou Medical University, Guangzhou, 510260, People’s Republic of China; 2Central Laboratory, The Second Affiliated Hospital, Guangzhou Medical University, Guangzhou, Guangdong, 510260, People’s Republic of China
Correspondence: Li Wan, Email [email protected]
Introduction: Allergic contact dermatitis (ACD), a prevalent skin disorder affecting up to 20% of the population, triggers significant discomfort and health implications. Our research investigates the pivotal role of Vascular Endothelial Growth Factor A (VEGFA) in chronic itching associated with ACD.
Methods: Bioinformatics methods were utilized to identify differentially expressed genes (DEGs) between ACD models and patients. In vivo models of chronic pruritus in mice induced by 2,4-dinitrofluorobenzene (DNFB) were employed. Mice were administered subcutaneously with a VEGFA inhibitor, sFlt1, and compared to a control group. Real-time RT-PCR, Western blot, and immunohistochemical staining were performed to evaluate VEGFA expression and the impact of sFlt1 on itching behavior.
Results: The analysis revealed that VEGFA is significantly upregulated in ACD skin, primarily expressed by keratinocytes. Administration of the VEGFA inhibitor sFlt1 in the ACD mouse model led to a substantial reduction in scratching behavior, indicating that VEGFA may mediate pruritus through the VEGFA-VEGFR2-PI3K-TRPV1 signaling pathway.
Discussion: These findings suggest that VEGFA plays a crucial role in ACD-associated pruritus and may serve as a potential therapeutic target. However, further research is required to validate these findings and to explore additional molecular pathways involved in the pruritic response in ACD.
Keywords: VEGFA, itch, allergic contact dermatitis, keratinocyte, VEGFR2
Introduction
Allergic contact dermatitis (ACD) is a common immunotoxic reaction to allergens in human skin that can cause significant discomfort and health issues.1 Multiple kinds of research have found that up to 20% of the total population is affected by ACD, making it the second most frequently reported occupational disease.2 ACD is characterized by a type IV delayed T-cell-mediated hypersensitivity to hapten, resulting in clinical symptoms of ACD, such as erythema and epidermospongiform lesions in the skin, and itching.3 Itching is the main manifestation of ACD and is an unpleasant experience that can cause scratching behavior and further health issues. Chronic and repeated itching can lead to significant health burdens for patients.4 Recent research on itch suggests that it primarily involves the transmission of signals between the environment, skin, immune cells, and afferent nerve fibers. These signals travel to the spinal circuits and to the brain, generating the sensation of itch and promoting scratching.5 In chronic itching caused by allergic contact dermatitis, the skin, as the first organ exposed to the allergen, plays a crucial role in the process, while the keratinocytes, as the initial living cells that come into contact with allergens in the skin, significantly contribute to the mechanisms underlying this condition.6
Keratinocyte is known to be a critical contributor to the development of ACD. After being stimulated, keratinocytes secrete cytokines, including interleukin 1 (IL-1), interleukin 6 (IL-6), and tumor necrosis factor (TNF-α), which promote the chemotaxis and inflammatory progression of T lymphocytes.7 Several recent studies have suggested that keratinocyte-mediated mechanisms are involved in the generation of pruritus.8 These mediators are characterized as opioids, proteases, substance P, nerve growth factor, neurotrophin 4, and endocannabinoids, and are known to activate various receptors implicated in the sensation of itching, such as PAR2 and TRPV ion channels, vanillin, Trk system potassium transporter A/B (TrkA/B), interleukin-31 (IL-31) receptor, cannabinoid receptor 1, and μ and κ opiate receptors. Such interactions point towards keratinocytes’ role as a pruritus initiator and also being responsible for transmitting itch signals to the cutaneous sensory C nerve.7–11 All of these indicate that keratinocytes play a pivotal role in the generation and transmission of the itch sensation. As such, this study takes keratinocytes as the primary focus to explore the potential role of keratinocytes in the itching of ACD.
Keratinocytes are key sources of vascular endothelial growth factor A (VEGFA), which is essential for vessel inflammation and keratinocyte function.12 This is particularly relevant in inflammatory skin conditions like psoriasis, where VEGFA promotes angiogenesis and exacerbates the disease’s pathogenesis.13,14 Inhibiting VEGFA, known to trigger keratinocyte hyperproliferation and inflammation, has been recognized as an effective therapeutic strategy for psoriasis and other inflammatory skin diseases.15 This underscores the importance of VEGFA in vascular remodeling and inflammation across various skin conditions, including contact dermatitis, atopic dermatitis, and UV-induced damage.16 Notably, allergic contact dermatitis is more common among patients with atopic dermatitis, where impaired skin barrier function facilitates the inflammatory impact of irritants or allergens.17 Additionally, dietary habits and smoking behaviors may exacerbate vascular inflammation, thereby increasing the risk of developing contact dermatitis.18,19 Nevertheless, in allergic contact dermatitis, the precise mechanisms through which VEGFA and keratinocytes exert their influence are not well understood, highlighting the need for more detailed research.
In order to investigate the role of keratinocytes in the development of allergic contact dermatitis (ACD), we conducted an analysis of transcriptomic data obtained from both ACD models and skin samples of ACD patients. The main objective of our research was to identify key genes that could potentially be targeted for therapeutic in ACD. To accomplish this, we employed several publicly available databases including GEO, KEGG, STRING, and GSEA. By conducting a comprehensive analysis of the data using the R programming language, we aim to provide novel insights and potential avenues for treatment of this debilitating condition.
In this study, significant differentially expressed genes were identified between keratinocytes of ACD models and the skin of stimulated ACD patients using bioinformatics methods. The analysis was done on the highly expressed VEGFA in keratinocytes of mice with ACD chronic pruritus which induced by 2, 4-dinitrofluorobenzene (DNFB). Further, the scratching responses to soluble vascular endothelial growth factor receptor 1 (sVEGFR1) - soluble FMS-like tyrosine kinase 1 (sFlt1) were observed by subcutaneous injection in ACD mice. And the pathway of VEGFA mediates DNFB-induced scratching was also investigated in ACD mice.
Materials and Methods
The Transcriptome Datasets
The gene expression datasets used in this study were obtained from the NCBI Gene Expression Omnibus (NCBI GEO) database (Accession Nos: GSE6281 and GSE76446). Both GSE7644620 and GSE628121 have been published (Table 1). These two data sets were generated using a microarray-based profiling approach, with laser capture microdissection used to extract RNA from formalin-treated human keratinocytes (KCs) following nickel sensitization, normal human KCs, and the skin of patients with nickel allergy.
 |
Table 1 Datasets Used in the Current Study |
Identification of Deferentially Expressed ACD Genes
Since both data sets were generated using the same platform, the same R package was utilized to import the expression data and to ensure more reliable data analysis. Specifically, the R data objects were generated from GSE76446 and GSE6281 using limma (v3.52.4). RMA (Robust multi-array average) method was employed for background adjustment, quantile normalization, summarization, and log2 scaling.22,23 The probes were annotated based on the accompanying GPL files. Non-matching probes (ie, probes with gene symbols that did not match) were removed from further analysis. For multiple probes that were mapped to the same gene symbol, their mean expression value was utilized for that gene symbol. Differential gene expression analysis was performed using limma (v3.52.4), with Student’s t-test used to calculate P-values for ACD with and without stimulation. Genes with |Fold change|> 1.5 and P < 0.05 were considered significant.
Functional Annotation and Enrichment Analysis on Common Differential Genes
The common upregulated and downregulated differentially expressed genes from both data sets were subjected to Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis using the R package clusterProfiler (v 4.4.4).24–26 The GO enrichment analysis included biological process (BP), cellular component (CC), and molecular function (MF) categories, with a focus on BP terms. Enrichment terms with a P-value < 0.05 were considered significant, with the minimum number of genes in each enriched term set to at least 3.
Protein–Protein Interaction (PPI) Network Analysis for Differential mRNA Expression Profile
To further analyze the common differentially expressed genes between the two data sets, we conducted protein-protein interaction (PPI) network analysis using the STRING Interactome database (v11.5).27 A low confidence score cutoff of 0.4 was set. The PPI network graph was constructed using Cytoscape (v3.6.0).28
Animals and Drugs
Male C57BL/6J mice, weight 20–25 g, 8 to 12 weeks of age, purchased from Guangdong Medical Laboratory Animal Center, were housed in 21 ± 1°C with a 12-h light/12-h dark room. All animal experiments were performed in accordance with the guidelines of the National Institutes of Health and the International Association for the Study of Pain and were approved by the Institutional Animal Care and Use Committee at Guangzhou Medical University (No. GY2019-007). The mouse VEGFR1 (sFlt1) recombinant protein was purchased from R&D Systems (China).
Generating a Mouse Model of ACD and Monitoring Scratching Behavior
The ACD mice model of chronic itch was induced by hapten 1-fluoro-2, 4-dinitrobenzene (DNFB). The hair of the abdomen and the nape of the neck were shaved 3 days before sensitization. Mice were sensitized with 100 μL of 0.15% DNFB onto the shaved abdominal skin. 5 days later, the mice were challenged with 50μL of 0.15% DNFB by painting the shaved nape of the neck, and then on days 3, 5, 7, and 9.29 Control mice (CTRL) were treated with acetone. The scratching behavior was video-recorded for 1 h on days 4, 6, 8, and 10. Bouts of scratching were counted in a blinded manner.
Drug Administration for the ACD Model
The study aimed to evaluate the role of VEGFA in male mice with ACD chronic itch through hypodermic administration of a selective VEGFA antagonist, sFlt1, or vehicle control with four mice per group. The mice were given hypodermic injections of 2 µg/kg of sFlt1 in 0.3% saline once daily on the shaved back on days 7, 8, 9, and 10 (total volume; 100µL). The scratching behavior was recorded video type for an hour before and after injection. Following behavioral testing, the mice were sacrificed and tissues were collected on the tenth day.
Western Blot Analysis of Total Proteins from Mouse Skin Tissue
The skin on the back of the neck was collected and homogenized in radio-immunoprecipitation assay buffer (RIPA, P0013B, Beyotime, Shanghai, China). Protein concentration was determined using a BCA protein assay kit. Equal amounts of total protein lysate were separated on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes. The membrane was blocked with 5% skim milk powder for 1 hour at room temperature and incubated overnight at 4°C with primary antibodies against VEGFA (1:2000, rabbit; Proteintech). As a loading control, the blots were probed with a β-actin antibody (1:5000, rabbit, Affinity). The membrane was washed three times with Tris-buffered saline in Tween 20 (TBST) and then incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:10000, Jackson ImmunoResearch) secondary antibodies for 1 hour at room temperature. After another three TBST washes, the membrane was visualized using an enhanced chemiluminescence (ECL) kit (Clinx). The band intensities were quantified using ImageJ and analyzed with GraphPad Prism 8.
Hematoxylin & Eosin (H&E) Staining for Epidermis Thickness Analysis
Paraffin-embedded skin tissues were obtained and utilized for immunohistochemistry (IHC) staining. Each tissue block was serially sectioned into 4 μm slices and mounted on glass slides, followed by baking at 65°C for 1 hour. The sections were deparaffinized using xylene and sequentially rehydrated with ethanol (from 95% to 50%). Hematoxylin staining was applied for 5 minutes followed by rinsing in distilled water and destaining with acid alcohol. After rinsing the sections in tap water, eosin counterstaining was performed for 2 minutes. The sections were then dehydrated with graded ethanol washes, cleared with xylene, and cover-slipped. Epidermal thickness was calculated by staining these sections with H&E and examining them under a magnification of 200x using Image Pro Plus software. Measurements were obtained from the left, medium, and right sections of each field.
Immune-Histochemical (IHC) Assay for VEGFA Semi-Quantitative Analysis
Tissue samples were fixed in 10% formalin and paraffin-embedded. The resulting paraffin blocks were sectioned into 4 μm slices and attached to glass slides. The sections were deparaffinized with xylene and rehydrated with a series of graded ethanol washes. Antigen retrieval was performed by boiling the sections in 10 mm sodium citrate buffer (pH 6.0, BOSTER) for 15 minutes, followed by blocking of endogenous peroxidase activity with 3% hydrogen peroxide for 10 minutes and 0.3% Triton-100 for 15 minutes at room temperature. The primary antibody for VEGFA (1:1000 dilution, rabbit; Proteintech) was left overnight at 4°C. After washing sections thrice with phosphate-buffered saline solution (PBS), a biotinylated goat anti-rabbit antibody (1:1000, Jackson ImmunoResearch) was added for 30 minutes. Finally, the skin was visualized with 3,3′-diaminobenzidine (DAB, Absin), counterstained with hematoxylin (Servicebio), dehydrated, and mounted. The IHC staining results were captured using a Leica scanning microscope (Olympus, Japan) and analyzed with Aperio ImageScope (Leica Biosystems) Profiler software.
RNA Isolation and Reverse Transcription Quantitative PCR (RT-qPCR)
Total RNA was extracted from fresh mice nape skin using TRIzol reagent (Invitrogen), followed by removal of genomic DNA. Reverse transcription was performed using the PrimeScript™ RT reagent Kit with gDNA Eraser (TAKARA) to obtain mRNA. Quantitative real-time PCR (qPCR) analysis was carried out using the SYBR® Premix Ex TaqTM II kit (TAKARA) and the Bio-Rad C1000 Thermal Cycler, following the manufacturer’s instructions. The sequences of the forward and reverse primers are provided in Table 2. The mRNA expression levels of the target genes were normalized to β-Actin and calculated using the 2−ΔΔCt method for data analysis.
 |
Table 2 The Common Upregulated and Downregulated DEGs Between GSE6281 and GSE76447 |
Statistical Analysis
All statistical analyses were performed using GraphPad Prism version 8.0 (GraphPad Software Inc., San Diego, CA). The data were presented as the mean ± standard error of the mean (SEM), and statistical significance was assessed using unpaired t-test for two groups. In cases of multiple comparisons, One-way analysis of variance (ANOVA) was conducted, followed by Fisher’s posthoc test, with a P-value of less than 0.05 was considered statistically significant.
Results
Genes Involved in Immune Response Emerged in Differentially Expressed Gene (DEG) Analysis
To investigate the role of keratinocytes (KCs) in the progression of ACD, we analyzed sequencing data from keratinocytes in an ACD model and skin samples from ACD patients. We used two mRNA microarray expression datasets sourced from the GEO database for our analysis. Table 1 summarizes the datasets used in this study, including their respective accession numbers, tissue sources, platforms utilized, and sample information. The first dataset was GSE76446, from which we selected four samples including two wild-type KCs and two formalin-stimulated keratinocytes. We compared and analyzed the transcriptome expression of the two groups. The second dataset was GSE6281, which included skin transcriptome data from ACD patients with nickel allergy. We selected six samples, including the skin transcriptome data of three ACD patients before nickel stimulation (0 hrs) and after nickel stimulation (96 hrs). We compared and analyzed the transcriptome expression of the two groups.
The differentially expressed gene (DEG) analysis was conducted using a threshold of FC > 1.5 expression and P value < 0.05. The expression of DEGs in the two datasets is illustrated in Figure 1A and C. For GSE76446, we identified 1362 DEGs; 706 genes were upregulated, and 656 genes were downregulated in formalin-stimulated KCS compared to wild-type KCS (Figure 1B). Similarly, for GSE6281, we identified 1218 DEGs, with 682 upregulated genes and 536 downregulated genes in nickel-stimulated skin compared to pre-stimulation skin (Figure 1D). The 20 most significant upregulated and downregulated genes with P<0.05, and with the highest FC, were analyzed for both datasets. We observed there were no statistically significant findings for the top 20 upregulated and downregulated genes in GSE76446. In contrast, among the upregulated genes in GSE6281, we identified genes related to immune responses that could be broadly categorized into three groups: (1) immune cell migration and chemotaxis genes, such as CCL18 and CCL27; (2) inflammatory response genes, such as TNFAIP6 and PLA2G2A; and (3) genes associated with the body’s defense response, such as IL7R and PLA2G2A. These results suggested that ACD may be associated with an immune response in KC cells and in ACD skin.
GO and KEGG Analyses Revealed Representative Genes Involved in the Recruitment of Immune Cells and Inflammation Factors in the Common Differentially Expressed Genes (DEGs) Found in KC Cells and ACD Skin
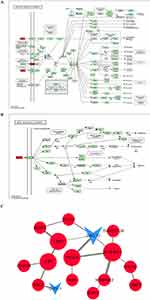
To further elucidate the role of KC cells in ACD skin, we identified 43 common DEGs between the GSE76446 and GSE6821 datasets. Among these genes, 23 were upregulated and 20 were downregulated (Figure 2A and Table 3). We propose that these genes represent the characteristics of key regulators of KC cells in ACD pathogenesis. Therefore, we focused on this set of intersecting DEGs in this study.
 |
Table 3 Primer Pairs’ Sequences of Mice Genes Used in RT-qPCR |
To gain functional insights into the DEGs involved in ACD, we performed functional enrichment analysis with GO and KEGG terms for co-upregulated and co-downregulated genes, respectively. However, GO term enrichment analysis on the downregulated genes did not yield any significant results. Thus, we focused on GO/KEGG terms enriched for upregulated genes (Figure 2B and C).
In the GO terms of upregulated genes, immune response-related genes were prominent, especially those involved in regulators of leukocyte chemotaxis and migration such as CD300A, CCL27, IL6R, SERPINE1, VEGFA, and RAC2 (Figure 2B). In KEGG terms, focal adhesion (including COL4A1, LAMC2, RAC2, and VEGFA) was related to cell attachment and migration. The AGE-RAGE signaling pathway (including COL4A1, SERPINE1, and VEGFA) is implicated in diabetic nephropathy.30 The HIF-1 signaling pathway (including IL6R, SERPINE1, and VEGFA) is a key factor in hypoxia stress and is associated with cell metastasis and chronic inflammation.31 The PI3K/Akt signaling pathway (including IL6R, LAMC2, VEGFA, and COL4A1; Figure 3A) is related to hormones, growth factors, and nutrient signaling. Dysregulations in this pathway can disturb cellular homeostasis, resulting in modifications to skin cell growth, proliferation, differentiation, and migration, while also promoting inflammation. This is closely associated with chronic pain and pruritus.32–35 Notably, VEGFR2 serves as the primary receptor for VEGFA, facilitating the activation of the PI3K-AKT pathway (Figure 3B).
Although CD300A, IL6R, SERPINE1, RAC2, LAMC2, and COL4A1 are included in the GO and KEGG terms, there is minimal literature linking these genes with ACD, and their potential roles in these conditions require further investigation. Conversely, CCL27, expressed in keratinocytes, interacts with CCR10, which leads to the chemotaxis of skin-associated memory T lymphocytes and contributes to their homing to skin sites, thus instigating skin inflammation in ACD.36,37 In addition, VEGFA was identified as a common factor in all KEGG-enriched pathways and GO/BP terms involved in the aforementioned processes. VEGFA has been shown to contribute to both lymphocyte chemotaxis and lymphangiogenesis during chronic inflammation,38 as well as to be associated with neuropathic pain39 and peripheral pain sensitization.40 Moreover, a positive correlation between elevated serum VEGFA levels and pruritus in ACD patients has been established.41 Notably, chemo-drugs that inhibit VEGFA have been shown to relieve pruritus symptoms in these patients.42
To understand potential protein-protein interactions, we constructed a PPI network using the common upregulated and downregulated DEGs between GSE76446 and GSE6821 with the STRING database. The network identified 17 interactions with a total of 16 nodes, where 14 nodes represented upregulated proteins and 2 nodes represented downregulated proteins (Figure 3C). The findings were consistent with the GO/KEGG enrichment results, showing that genes encoding key regulatory factors implicated in leukocyte chemotaxis and migration were also enriched in the most interactive modules. In summary, VEGFA is involved in all significantly enriched GO/BP and KEGG terms enriched by upregulated DEGs, which was also confirmed by the PPI enrichment analysis. Given these results, it would appear that VEGFA plays a crucial role in the pathogenesis of ACD, specifically the symptom of pruritus. Therefore, our research will concentrate on VEGFA and its associated pathways to further elucidate their potential role in pruritus development in ACD chronic itch.
VEGFA is Highly Expressed in the Skin of the ACD Mouse Model
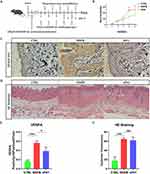
To investigate the potential role of VEGFA in the pathogenesis of ACD, we utilized a mouse model of chronic itching induced by DNFB. The control group was treated with the same dose of DNFB solvent acetone, as illustrated in Figure 4A. We observed a gradually increased scratching behavior from the second DNFB challenge, reaching its peak after the fourth challenge and then gradually decreased (Figure 4B), which is consistent with our previous findings of DNFB-induced ACD chronic itching model.43 After the final video recording, skin samples were collected, and protein was extracted for subsequent Western blot analysis. The results revealed a significantly increased VEGFA expression in the DNFB group, as shown in Figure 4C and D. Statistical analysis indicated that the difference in VEGFA expression between the DNFB and control groups was very significant (P < 0.0001). These findings suggest that VEGFA may be involved in the development of chronic ACD pruritus induced by DNFB.
The VEGFA Inhibitor sFlt1 Inhibited Itching Behavior in an ACD Mouse Model but Did Not Alleviate Skin Thickness
To further investigate the potential role of VEGFA in chronic itching, we conducted subcutaneous injections of the VEGFA inhibitor sFlt1 in ACD mouse models. Given that the mice did not display peak itching behavior until the fourth challenges, we administered the drug four times over four consecutive days, with injections given one hour before and one hour after for video recordings, respectively, following the fourth challenges. The injection timeline is illustrated in Figure 5A. To serve as controls, the DNFB group and control (CTRL) group mice were given the same volume of normal saline at the same time intervals as the sFlt1 and VEGFA groups. We then monitored the pruritus behavior of the mice using a behavioral observation cage. Interestingly, administration of sFlt1 resulted in a significant reduction of the number of scratching when compared to the DNFB group (Figure 5B), indicating that VEGFA may indeed play a key role in the development of chronic ACD pruritus. After the final video recording, both Hematoxylin and Eosin (HE) staining and immunohistochemistry were performed to investigate the expression of VEGFA in the skin. Remarkably, we found that the DNFB group exhibited significantly higher VEGFA expression compared to both the sFlt1 and CTRL groups (Figure 5C-E). Furthermore, it revealed that VEGFA expression was predominantly located in the KC cells of the epidermis, providing additional evidence that these cells may play a critical role in ACD through VEGFA secretion (Figure 5C). Consistent with this idea, we also found a significantly increased VEGFA expression in the DNFB group when compared with the CTRL and sFlt1 groups (Figure 5C-E). Interestingly, we observed that the skin of the DNFB group was considerably thicker than that of the CTRL group, but there was no significant difference between the DNFB group and sFlt1 groups (Figure 5D and F). Our results suggest that VEGFA may promote pruritus in the skin, but is not involve in the epidermal thickening processes associated with ACD.
VEGFA-VEGFR2-PI3K-TRPV1 Pathway Identified as a Promoter of Pruritus in Chronic Skin Itching
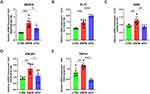
To investigate the potential involvement of VEGFA in pruritus associated with ACD, we performed real-time RT-PCR to measure VEGFA mRNA expression in skin following the DNFB challenge. Our results demonstrated a statistically significantly increased (2-fold) VEGFA mRNA expression in DNFB-treated skin as compared to control skin (Figure 6A). Surprisingly, the soluble VEGFR1 recombinant protein -sFlt1, a VEGFA inhibitor, led to a noticeable reduction in VEGFA mRNA expression in the sFlt1 group. Moreover, the expression of FLT1 was higher in the DNFB group when compared to the CTRL group, but significantly higher in the sFlt1 group than that of the DNFB group (Figure 6B). These aberrant expression patterns of VEGFA and FLT1 suggest a possibly additional mechanisms regulate their expression, such as post-transcriptional modifications, which may further suppress their expression in the skin.
The functional potency of VEGFA primarily relies on its interaction with and activation of VEGFR2 (Figure 3B), a downstream receptor crucial for transmitting its biological effects.44,45 Furthermore, PI3K is commonly downstream of VEGFR2 and plays a role in promoting cell survival and chemotaxis.46 The maintenance of skin homeostasis relies heavily on the proper functioning of the PI3K pathway. Dysregulation of this pathway can lead to the development of various benign or malignant skin diseases, and the PI3K pathway plays a crucial role in the progression of inflammatory skin diseases such as psoriasis and atopic dermatitis.33,47,48 As an upstream regulator of PI3K, VEGFA’s role in tumor progression is well-established,49 and recent studies have also begun to explore its role in pain regulation.39 Notably, KDR and PIK3R1 expressions were significantly higher in the DNFB group than that in the CTRL group (Figure 6C and D), but were downregulated following VEGFA inhibition, implying that VEGFR2 and PI3K may be regulated by VEGFA in skin, which is consistent with our signaling results (Figure 3B). TRPV1 has been extensively studied as a key pathway in itch, as demonstrated by numerous articles.50–52 As the downstream of PI3K, TRPV1 is involved in various diseases like skin inflammation and neuropathic pain.53,54 In our study, TRPV1 expression was significantly elevated in the DNFB group when compared to the CTRL and sFlt1 groups (Figure 6E), suggesting that sFlt1 might have reduced TRPV1 expression by neutralizing VEGFA, thereby mitigating the itch sensation in the ACD mouse model. Altogether, our study suggest that VEGFA participates in ACD chronic itch through the VEGFR2/PI3K/TRPV1 axis that can be suppressed by the VEGFA inhibitor sFlt1.
In conclusion, VEGFA involves in chronic itching processes through the secretion of VEGFA in the ACD mouse model and may serve as a potential therapeutic target for treating chronic itching in ACD patients.
Discussion
This study aimed to investigate allergic contact dermatitis (ACD) and its associated pruritus. The risk factors for ACD can be either acquired or innate. Acquired risk factors include pre-existing inflammatory skin conditions such as irritant contact dermatitis (ICD) and stasis dermatitis, both of which contribute to ACD development by compromising the skin barrier. Innate risk factors encompass genetic predispositions, such as mutations in the gene encoding filaggrin—a protein crucial for epidermal differentiation and skin barrier function.55 Notably, allergic contact dermatitis is particularly prevalent among atopic dermatitis patients due to impaired skin barrier function, which facilitates the inflammatory impact of irritants or allergens, with VEGFA playing a crucial role in the related inflammation subsequent to skin barrier injury.17,56,57 Therefore, maintaining skin barrier integrity and mitigating inflammation from subsequent allergen exposure is essential in managing ACD. This is particularly important in occupations with higher exposure to irritants or allergens.58–60 Additionally, many patients require pharmacological interventions to alleviate pruritus. Recent studies have demonstrated the efficacy of dupilumab, a JAK inhibitor, in treating AD by targeting JAK to reduce symptoms.61–63 This study also seeks to identify targeted therapies, similar to dupilumab, for the treatment of ACD, with a focus on VEGFA as a potential therapeutic target.
Recent studies have shown that VEGFA plays a crucial role in the nervous system.64,65 VEGFA is a dimeric secretory protein that is upregulated in response to hypoxia.66 In the peripheral nervous system, VEGFA can induce axon growth and survival of sensory neurons, leading to sensory sensitization and exacerbation of pruritus.67 Furthermore, VEGF is also involved in angiogenesis and inflammation, making it a potential target for diseases involving inflammation.68,69 Additionally, VEGFA has been linked to pruritus in several studies. Patients with atopic dermatitis (AD) have significantly elevated levels of VEGF in serum.70 Similarly, a positive correlation between VEGF-A levels in the serum of patients with mycosis fungoides (MF) and Sézary syndrome (SS), and pruritus markers has been observed. These findings imply that VEGFA might have a role in pruritus.41 Moreover, a case report by Krause showed a significant improvement in pruritus symptoms in a patient with prurigo simplex after treatment with bevacizumab, a monoclonal antibody targeting VEGF.42 Indeed, several studies have suggested that inhibiting VEGFA may have the potential to alleviate pruritus symptoms in patients. However, the source of VEGFA remains largely unknown, and only a few studies have reported elevated levels of VEGFA in the skin of patients.42 So, we hypothesize that VEGFA is derived from the skin and is derived from the keratinocytes in the skin. This hypothesis has been confirmed in our bioinformatic analysis and experiment, which is consistent with clinical case reports. Furthermore, above studies provide evidence for the involvement of VEGFA in pruritus and the potential for symptom alleviation through VEGFA inhibition.
Find out the mechanisms underlying VEGFA-induced pruritus requires further exploration and experimental validation is necessary. In this study, we established a DNFB-induced chronic pruritus mouse model to continue investigating the potential involvement of VEGFA in ACD. Our findings demonstrated a significant reduction in pruritic behavior in ACD mice when the expression of VEGFA was inhibited using the inhibitor sFlt1. Moreover, PCR experiments provided additional evidence supporting the notion that sFlt1 may alleviate pruritus in ACD mice by inhibiting the VEGFA-VEGFR2-PI3K-TRPV1 pathway. Nonetheless, there are certain experimental results that require further discussion. Firstly, we confirmed that VEGFA is mainly expressed in the epidermis, with keratinocytes being the primary cell type.71 However, relying solely on the above experimental evidence seems somewhat inadequate to suggest that keratinocytes secrete VEGFA to promote chronic ACD pruritus. Further experiments such as fluorescence co-staining of keratinocytes with VEGFA or flow cytometry will be necessary to provide additional evidence. Secondly, our qRT-PCR results showed that VEGFA mRNA expression was downregulated in the sFlt1 group compared to the DNFB group, while VEGFR1 mRNA expression was significantly upregulated. Nonetheless, these findings require validation at the protein level through additional experiments.
It is important to note that sFlt1, also known as soluble vascular endothelial growth factor receptor 1 (VEGFR1), functions as both a high-affinity receptor for VEGFA and a naturally occurring protein. Due to its properties, it serves as an ideal antagonist for VEGFA.46,49 The sFlt1 reduces the expression of VEGFR2 by neutralizing VEGFA. Additionally, it directly interacts with VEGFR2 to block its activity, resulting in sVEGFR-1 exhibiting anti-angiogenesis, anti-edema, and anti-inflammatory effects.72,73 In this experiment, the administration of sFlt1 alleviated the pathological state of the skin, leading to a decrease in the mRNA expression of VEGFA. Furthermore, the observed higher mRNA expression level of VEGFR1 in the sFlt1 group compared to the DNFB group may be attributed to potential post-transcriptional modifications or involvement of another signaling pathway. Interestingly, a study on ovarian cancer suggested a positive feedback loop between VEGFA and the autotaxin (ATX) - lysophosphatidic acid (LPA) axis. Specifically, VEGFA can enhance the expression of ATX-LPA, and inhibition of ATX leads to decreased expression of LPA and VEGFR2, while increasing VEGFR1 expression.74 This suggests that in certain cases, the expression of VEGFA and VEGFR1 may be inversely related. Further exploration is required to better understand the role of these mechanisms in the context of skin biology.
The sole focus on VEGFA leaves the roles of other DEGs unaddressed. Elucidation of upstream regulation and full signaling mechanisms is still needed. Hypoxia-inducible factor (HIF-1) pathway, a critical stress pathway involved in multiple diseases, was significantly enriched.75,76 HIF-1 acts as an upstream transcription factor of VEGFA and promotes its secretion under conditions of tissue hypoxia stress.66,77,78 Furthermore, we observed that the PI3K-AKT pathway, which is a crucial regulator of angiogenesis and inflammation, was also enriched, underscoring the potential of VEGFA as an upstream factor of this pathway.79 Therefore, VEGFA may play a key connecting role in the pathogenesis of ACD. During the initial stages of ACD, we propose that the interaction between external allergens and inflammatory factors induces the proliferation of keratinocytes, leading to the thickening of the epidermis, particularly within the spinous layer. As the keratinocytes move closer to the outer layer, they are susceptible to hypoxic conditions due to limited nutrient transport. This activation of the HIF-1 hypoxia stress pathway subsequently increases VEGFA secretion. VEGFA, in turn, promotes the recruitment of inflammatory cells, aggravates skin inflammation through downstream activation of the PI3K-AKT pathway, and also directly stimulates peripheral sensory nerves, resulting in sensory nerve sensitization and exacerbating pruritus. Future studies should examine other identified DEGs, and trial VEGFA inhibition in patients to relieve ACD-associated pruritus.
To summarize, our bioinformatics analysis suggested that keratinocytes may contribute to the development of ACD by secreting VEGFA. Our study provided evidence that VEGFA is involved in DNFB-induced pruritus in mice and may be involved in DNFB-induced pruritus in ACD mice through the VEGFA-VEGFR2-PI3K-TRPV1 axis. Inhibiting VEGFA expression significantly reduced scratching behavior in ACD mice. Therefore, targeting VEGFA may be a promising therapeutic strategy for treating pruritus in ACD patients. Future studies should focus on exploring the underlying molecular mechanisms involved in the interaction between VEGFA and pruritus, as well as investigating the possibility of using VEGFA inhibitors as a safe and effective treatment for pruritus in ACD patients.
Ultimately, these efforts will not only deepen our understanding of the pathogenesis of pruritus in ACD but also lead to the development of novel therapeutic strategies to alleviate this distressing symptom and improve the quality of life of affected patients.
Conclusion
In conclusion, this study identifies VEGFA as a key contributor to chronic pruritus in allergic contact dermatitis (ACD), primarily through the VEGFA-VEGFR2-PI3K-TRPV1 pathway. Inhibiting VEGFA significantly reduced itching behavior in ACD mouse models, highlighting its potential as a therapeutic target. However, the study has limitations, including the need for further validation of VEGFA’s role at the protein level and more detailed exploration of other differentially expressed genes. Further research should investigate these mechanisms to enhance our understanding of ACD pathogenesis and improve treatment options.
Ethical Statement
This study is exempt from ethical review and is in accordance with Article 32 of the “Notice on Issuing the Measures for Ethical Review of Life Sciences and Medical Research Involving Humans” (revised in 2023) of the People’s Republic of China, which states that “for life sciences and medical research involving humans that uses human information data or biological samples in the following situations, ethical review may be waived if it does not cause harm to human subjects, does not involve sensitive personal information or commercial interests, in order to reduce unnecessary burdens on researchers and promote the conduct of life sciences and medical research involving humans”. This study falls under the first item: “research using publicly available data obtained legally, or data generated through observation without interfering with public behavior”.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81771182) to Li Wan and by the High-level University Construction Promotion Project of Guangzhou Medical University (2017-No.106-9).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin North Am. 2020;104(1):61–76. doi:10.1016/j.mcna.2019.08.012
2. Olusegun OA, Martincigh BS. Allergic contact dermatitis: a significant environmental and occupational skin disease. Int J Dermatol. 2021;60(9):1082–1091. doi:10.1111/ijd.15502
3. Scheinman PL, Vocanson M, Thyssen J.P, et al. Contact dermatitis. Nat Rev Dis Primers. 2021;7(38).
4. Buddenkotte J, Steinhoff M. Pathophysiology and therapy of pruritus in allergic and atopic diseases. Allergy. 2010;65(7):805–821. doi:10.1111/j.1398-9995.2010.01995.x
5. Cevikbas F, Lerner EA. Physiology and Pathophysiology of Itch. Physiol Rev. 2020;100(3):945–982. doi:10.1152/physrev.00017.2019
6. Moniaga C, Tominaga M, Takamori K. Mechanisms and management of itch in dry skin. Acta Derm Venerol. 2020;100(2):10–21. doi:10.2340/00015555-3344
7. Barker JNWA. Role of keratinocytes in allergic contact dermatitis. Contact Dermatitis. 1992;26(3):145–148. doi:10.1111/j.1600-0536.1992.tb00283.x
8. Zhao J, Munanairi A, Liu X-Y, et al. PAR2 mediates itch via TRPV3 signaling in keratinocytes. J Invest Dermatol. 2020;140(8):1524–1532. doi:10.1016/j.jid.2020.01.012
9. Geppetti P, Veldhuis NA, Lieu T, Bunnett NW. G protein-coupled receptors: dynamic machines for signaling pain and itch. Neuron. 2015;88(4):635–649. doi:10.1016/j.neuron.2015.11.001
10. Mollanazar NK, Smith PK, Yosipovitch G. Mediators of chronic pruritus in atopic dermatitis: getting the itch out? Clin Rev Allerg Immunol. 2016;51:263–292.
11. Mack MR, Kim BS. The itch–scratch cycle: a neuroimmune perspective. Trends Immunol. 2018;39(12):980–991. doi:10.1016/j.it.2018.10.001
12. Bae O-N, Noh M, Chun Y-J, Jeong TC. Keratinocytic vascular endothelial growth factor as a novel biomarker for pathological skin condition. Biomol Ther. 2015;23(1):12–18. doi:10.4062/biomolther.2014.102
13. Zhou X, Chen Y, Cui L, Shi Y, Guo C. Advances in the pathogenesis of psoriasis: from keratinocyte perspective. Cell Death Dis. 2022;13(1):81. doi:10.1038/s41419-022-04523-3
14. Benhadou F, Glitzner E, Brisebarre A, et al. Epidermal autonomous VEGFA/Flt1/Nrp1 functions mediate psoriasis-like disease. Sci Adv. 2020;6(2):eaax5849. doi:10.1126/sciadv.aax5849
15. Chen Y, Tai Z, Zhu C, et al. Vascular endothelial growth factor A VEGFA inhibition: an effective treatment strategy for psoriasis. Int J Mol Sci. 2023;25(1):59. doi:10.3390/ijms25010059
16. Eming SA, Krieg T. Molecular mechanisms of VEGF-A action during tissue repair. J Invest Dermatol Symp Proc. 2006;11(1):79–86. doi:10.1038/sj.jidsymp.5650016
17. Damiani G, Calzavara‐Pinton P, Stingeni L, et al. Italian guidelines for therapy of atopic dermatitis-Adapted from consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis). Dermatol Ther. 2019;32(6):e13121. doi:10.1111/dth.13121
18. Bragazzi NL, Sellami M, Salem I, et al. Fasting and its impact on skin anatomy, physiology, and physiopathology: a comprehensive review of the literature. Nutrients. 2019;11(2):249. doi:10.3390/nu11020249
19. Kendrick PJ, Reitsma MB, Abbasi-Kangevari M; GBD 2019. Chewing tobacco collaborators. spatial, temporal, and demographic patterns in prevalence of chewing tobacco use in 204 countries and territories, 1990-2019: a systematic analysis from the global burden of disease study 2019. Lancet Public Health. 2021;6(7):e482–e499. doi:10.1016/S2468-2667(21)00065-7
20. Lee E, Kim H-J, Lee M, et al. Cystathionine metabolic enzymes play a role in the inflammation resolution of human keratinocytes in response to sub-cytotoxic formaldehyde exposure. Toxicol Appl Pharmacol. 2016;310:185–194. doi:10.1016/j.taap.2016.09.017
21. Pedersen MB, Skov L, Menné T, Johansen JD, Olsen J. Gene expression time course in the human skin during elicitation of allergic contact dermatitis. J Invest Dermatol. 2007;127(11):2585–2595. doi:10.1038/sj.jid.5700902
22. Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19(2):185–193. doi:10.1093/bioinformatics/19.2.185
23. Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31(4):e15. doi:10.1093/nar/gng015
24. Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. doi:10.1093/nar/28.1.27
25. Yu G, Wang L-G, Han Y, He Q-Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16(5):284–287. doi:10.1089/omi.2011.0118
26. The Gene Ontology Consortium. The gene ontology resource: 20 years and still going strong. Nucleic Acids Res. 2019;47(D1):D330–D338. doi:10.1093/nar/gky1055
27. Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–D613. doi:10.1093/nar/gky1131
28. Kohl M, Wiese S, Warscheid B. Cytoscape: software for visualization and analysis of biological networks. Methods Mol Biol. 2011;696:291–303.
29. Zhao Z-Q, Huo F-Q, Jeffry J, et al. Chronic itch development in sensory neurons requires BRAF signaling pathways. J Clin Invest. 2013;123(11):4769–4780. doi:10.1172/JCI70528
30. Wu X-Q, Zhang -D-D, Wang Y-N, et al. AGE/RAGE in diabetic kidney disease and ageing kidney. Free Radic Biol Med. 2021;171:260–271. doi:10.1016/j.freeradbiomed.2021.05.025
31. Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science. 2016;352(6282):175–180. doi:10.1126/science.aaf4405
32. Liu W, Lv Y, Ren F. PI3K/Akt pathway is required for spinal central sensitization in neuropathic pain. Cell Mol Neurobiol. 2018;38(3):747–755. doi:10.1007/s10571-017-0541-x
33. Chen S-P, Zhou Y-Q, Liu D-Q, et al. PI3K/Akt pathway: a potential therapeutic target for chronic pain. CPD. 2017;23(12):1860–1868. doi:10.2174/1381612823666170210150147
34. Le M, Ng C, Na J. Itchy mice: the identification of a new pathway for the development of autoimmunity. Curr Top Microbiol Immunol. 2008;321.
35. Dixelius J, Olsson A-K, Thulin A, et al. Minimal active domain and mechanism of action of the angiogenesis inhibitor histidine-rich glycoprotein. Cancer Res. 2006;66(4):2089–2097. doi:10.1158/0008-5472.CAN-05-2217
36. Homey B, Alenius H, Müller A, et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8(2):157–165. doi:10.1038/nm0202-157
37. Kanda N, Koike S, Watanabe S. IL-17 suppresses TNF-alpha-induced CCL27 production through induction of COX-2 in human keratinocytes. J Allergy Clin Immunol. 2005;116(5):1144–1150. doi:10.1016/j.jaci.2005.08.014
38. Halin C, Tobler NE, Vigl B, Brown LF, Detmar M. VEGF-A produced by chronically inflamed tissue induces lymphangiogenesis in draining lymph nodes. Blood. 2007;110(9):3158–3167. doi:10.1182/blood-2007-01-066811
39. Peng Z, Yang F, Huang S, Tang Y, Wan L. Targeting Vascular endothelial growth factor A with soluble vascular endothelial growth factor receptor 1 ameliorates nerve injury-induced neuropathic pain. Mol Pain. 2022;18:174480692210945. doi:10.1177/17448069221094528
40. Kiguchi N, Kobayashi Y, Kadowaki Y, et al. Vascular endothelial growth factor signaling in injured nerves underlies peripheral sensitization in neuropathic pain. J Neurochem. 2014;129(1):169–178. doi:10.1111/jnc.12614
41. Sakamoto M, Miyagaki T, Kamijo H, et al. Serum vascular endothelial growth factor A levels reflect itch severity in mycosis fungoides and Sézary syndrome. J Dermatol. 2018;45(1):95–99. doi:10.1111/1346-8138.14033
42. Krause K, Krull C, Kessler B, et al. Effective control of recalcitrant pruritus by bevacizumab: a possible role for vascular endothelial growth factor in chronic itch? Acta Derm Venerol. 2013;93(2):175–179. doi:10.2340/00015555-1445
43. Paluch EK, Aspalter IM, Sixt M. Focal adhesion-independent cell migration. Annu Rev Cell Dev Biol. 2016;32(1):469–490. doi:10.1146/annurev-cellbio-111315-125341
44. Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176(6):1248–1264. doi:10.1016/j.cell.2019.01.021
45. Peach CJ, Mignone VW, Arruda MA, et al. Molecular pharmacology of VEGF-A isoforms: binding and signalling at VEGFR2. Int J Mol Sci. 2018;19(4):1264. doi:10.3390/ijms19041264
46. Ferrara N, Gerber H-P, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi:10.1038/nm0603-669
47. Teng Y, Fan Y, Ma J, et al. The PI3K/Akt pathway: emerging roles in skin homeostasis and a group of non-malignant skin disorders. Cells. 2021;10(5):1219. doi:10.3390/cells10051219
48. Macdonald JB, Macdonald B, Golitz LE, LoRusso P, Sekulic A. Cutaneous adverse effects of targeted therapies: part II: inhibitors of intracellular molecular signaling pathways. J Am Acad Dermatol. 2015;72(2):221–236. doi:10.1016/j.jaad.2014.07.033
49. Claesson-Welsh L, Welsh M. VEGFA and tumour angiogenesis. J Intern Med. 2013;273(2):114–127. doi:10.1111/joim.12019
50. Bánvölgyi Á, Pálinkás L, Berki T, et al. Evidence for a novel protective role of the vanilloid TRPV1 receptor in a cutaneous contact allergic dermatitis model. J Neuroimmunol. 2005;169(1–2):86–96. doi:10.1016/j.jneuroim.2005.08.012
51. Meng J, Li Y, Fischer MJM, et al. Th2 modulation of transient receptor potential channels: an unmet therapeutic intervention for atopic dermatitis. Front Immunol. 2021;12:696784. doi:10.3389/fimmu.2021.696784
52. Feng J, Yang P, Mack MR, et al. Sensory TRP channels contribute differentially to skin inflammation and persistent itch. Nat Commun. 2017;8(1):980. doi:10.1038/s41467-017-01056-8
53. Stratiievska A, Nelson S, Senning EN, et al. Reciprocal regulation among TRPV1 channels and phosphoinositide 3-kinase in response to nerve growth factor. Elife. 2018;7:e38869. doi:10.7554/eLife.38869
54. Fang D, Kong L-Y, Cai J, et al. Interleukin-6-mediated functional upregulation of TRPV1 receptors in dorsal root ganglion neurons through the activation of JAK/PI3K signaling pathway: roles in the development of bone cancer pain in a rat model. Pain. 2015;156(6):1124–1144. doi:10.1097/j.pain.0000000000000158
55. Peiser M, Tralau T, Heidler J, et al. Allergic contact dermatitis: epidemiology, molecular mechanisms, in vitro methods and regulatory aspects. Current knowledge assembled at an international workshop at BfR, Germany. Cell Mol Life Sci. 2012;69(5):763–781. doi:10.1007/s00018-011-0846-8
56. Milam EC, Jacob SE, Cohen DE. Contact dermatitis in the patient with atopic dermatitis. J Allergy Clin Immunol Pract. 2019;7(1):18–26. doi:10.1016/j.jaip.2018.11.003
57. Birkenhauer E, Neethirajan S. A double-edged sword: the role of VEGF in wound repair and chemoattraction of opportunist pathogens. Int J Mol Sci. 2015;16(4):7159–7172. doi:10.3390/ijms16047159
58. Belsito DV. The diagnostic evaluation, treatment, and prevention of allergic contact dermatitis in the new millennium. J Allergy Clin Immunol. 2000;105(3):409–420. doi:10.1067/mai.2000.104937
59. Silverberg NB, Pelletier JL, Jacob SE, Schneider LC. Nickel allergic contact dermatitis: identification, treatment, and prevention. Pediatrics. 2020;145(5):e20200628. doi:10.1542/peds.2020-0628
60. Fyhrquist N, Lehto E, Lauerma A. New findings in allergic contact dermatitis. Curr Opin Allergy Clin Immunol. 2014;14(5):430–435. doi:10.1097/ACI.0000000000000092
61. Hagino T, Hamada R, Yoshida M, et al. Effectiveness and safety of upadacitinib in combination with topical corticosteroids in adolescent patients with moderate-to-severe atopic dermatitis. Clin Cosmet Invest Dermatol. 2023;16:3201–3212. doi:10.2147/CCID.S439053
62. Hagino T, Yoshida M, Hamada R, et al. Effectiveness of switching from baricitinib 4 mg to upadacitinib 30 mg in patients with moderate-to-severe atopic dermatitis: a real-world clinical practice in Japan. J Dermatological Treat. 2023;34(1). doi:10.1080/09546634.2023.2276043
63. Hagino T, Hamada R, Yoshida M, et al. Total eosinophil count as a biomarker for therapeutic effects of upadacitinib in atopic dermatitis over 48 weeks. Front Immunol. 2024;15:1365544. doi:10.3389/fimmu.2024.1365544
64. Moon S, Chang M-S, Koh S-H, Choi YK. Repair mechanisms of the neurovascular unit after ischemic stroke with a focus on VEGF. Int J Mol Sci. 2021;22(16):8543. doi:10.3390/ijms22168543
65. Theis V, Theiss C. VEGF - A stimulus for neuronal development and regeneration in the CNS and PNS. Curr Protein Pept Sci. 2018;19(6):589–597. doi:10.2174/1389203719666180104113937
66. Lee JW, Ko J, Ju C, Eltzschig HK. Hypoxia signaling in human diseases and therapeutic targets. Exp Mol Med. 2019;51(6):68. doi:10.1038/s12276-019-0235-1
67. Cattin A-L, Burden JJ, Van Emmenis L, et al. Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell. 2015;162(5):1127–1139. doi:10.1016/j.cell.2015.07.021
68. Kirk SL, Karlik SJ. VEGF and vascular changes in chronic neuroinflammation. J Autoimmun. 2003;21(4):353–363. doi:10.1016/S0896-8411(03)00139-2
69. Shibuya M. VEGF-VEGFR system as a target for suppressing inflammation and other diseases. Endocr Metab Immune Disord Drug Targets. 2015;15(2):135–144. doi:10.2174/1871530315666150316121956
70. Gomułka K, Wójcik E, Szepietowski JC. Serum levels of eosinophil-derived neurotoxin, platelet-activating factor and vascular endothelial growth factor in adult patients with atopic dermatitis-a pilot study. Biomedicines. 2022;10(12):3109. doi:10.3390/biomedicines10123109
71. Eckert RL, Crish JF, Robinson NA. The epidermal keratinocyte as a model for the study of gene regulation and cell differentiation. Physiol Rev. 1997;77(2):397–424. doi:10.1152/physrev.1997.77.2.397
72. Nevo O, Lee DK, Caniggia I. Attenuation of VEGFR-2 expression by sFlt-1 and low oxygen in human placenta. PLoS One. 2013;8(11):e81176. doi:10.1371/journal.pone.0081176
73. Ceci C, Atzori MG, Lacal PM, Graziani G. Role of VEGFs/VEGFR-1 signaling and its inhibition in modulating tumor invasion: experimental evidence in different metastatic cancer models. Int J Mol Sci. 2020;21(4):1388. doi:10.3390/ijms21041388
74. Ptaszynska MM, Pendrak ML, Bandle RW, Stracke ML, Roberts DD. Positive feedback between vascular endothelial growth factor-A and autotaxin in ovarian cancer cells. Mol Cancer Res. 2008;6(3):352–363. doi:10.1158/1541-7786.MCR-07-0143
75. Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40(2):294–309. doi:10.1016/j.molcel.2010.09.022
76. Gunton JE. Hypoxia-inducible factors and diabetes. J Clin Invest. 2020;130(10):5063–5073. doi:10.1172/JCI137556
77. Campochiaro PA. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog Retin Eye Res. 2015;49:67–81. doi:10.1016/j.preteyeres.2015.06.002
78. Zimna A, Kurpisz M. Hypoxia-inducible factor-1 in physiological and pathophysiological angiogenesis: applications and therapies. Biomed Res Int. 2015;2015:549412. doi:10.1155/2015/549412
79. Chen M, Zhong K, Tan J, et al. Baicalein is a novel TLR4-targeting therapeutics agent that inhibits TLR4/HIF-1α/VEGF signaling pathway in colorectal cancer. Clin Transl Med. 2021;11(11):e564. doi:10.1002/ctm2.564
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.