Back to Journals » Journal of Inflammation Research » Volume 17
Visceral Adiposity and Neutralizing Antibody Expression: An Adult-Based Cross-Sectional Study
Authors Wang H, Xiao D, Zhou H, Chen S, Xiao G , Hu J, Quan H, Luo M, Zhang S
Received 9 May 2024
Accepted for publication 16 August 2024
Published 26 August 2024 Volume 2024:17 Pages 5633—5643
DOI https://doi.org/10.2147/JIR.S477526
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Huanhuan Wang,1,* Dan Xiao,2,* Hua Zhou,3,* Shu Chen,4 Guangjun Xiao,4 Juan Hu,4 Hui Quan,2 Miao Luo,5 Shaocheng Zhang2,6
1School of Laboratory Medicine, Chengdu Medical College, Chengdu, Sichuan, People’s Republic of China; 2Department of Laboratory Medicine, the Second Affiliated Hospital of Chengdu Medical College (Nuclear Industry 416 Hospital), Chengdu, Sichuan, People’s Republic of China; 3Department of Laboratory Medicine, the Second Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China; 4Department of Laboratory Medicine, Suining Central Hospital, Suining, Sichuan, People’s Republic of China; 5Department of Laboratory Medicine, the People’s Hospital of Yubei District of Chongqing, Chongqing, People’s Republic of China; 6School of Clinical Medicine, Chengdu Medical College, Chengdu, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shaocheng Zhang; Miao Luo, Email [email protected]; [email protected]
Purpose: Visceral adiposity is a significant risk factor for severe COVID-19. However, the impact of the Chinese visceral adiposity index (CVAI) on the efficacy of SARS-CoV-2 vaccines remains poorly understood. This study aims to explore the impact of CVAI on the production of neutralizing antibodies (NAb) in inactivated SARS-CoV-2 vaccines and the potential mechanism, thereby optimizing vaccination guidance.
Methods: In this cross-sectional study, 206 health workers (completed two SARS-CoV-2 vaccination on February 8th and March 10th, 2021, respectively) were recruited. All baseline anthropometric parameters of the participants were collected, and venous blood samples were obtained 6 weeks later to measure peripheral innate immune cells, inflammatory cytokines, and NAb titers against SARS-CoV-2. CVAI were calculated according to the formula and divided participants into two groups depending on CVAI median.
Results: The median NAb titer among healthcare workers was 12.94 AU/mL, with an efficacy of 87.86% for the SARS-CoV-2 vaccine. NAb titers were lower in the CVAI dysfunction group than in the CVAI reference group (median: 11.40 AU/mL vs 15.57 AU/mL), the hsCRP levels (median: 0.50 mg/L vs 0.30 mg/L) and peripheral monocyte count (mean: 0.47 × 109/L vs 0.42 × 109/L) in the CVAI dysfunction group were higher than in the CVAI reference group. Additionally, CVAI showed positive correlations with hsCRP, monocytes, lymphocytes, and B-lymphocytes, and a negative correlation with NAb titers.
Conclusion: CVAI may inhibit SARS-CoV-2 neutralizing antibody expression through inducing immune dysfunction and chronic inflammation. Thus, more attention should be paid to the vaccination for high CVAI population to improve the effectiveness of vaccination, which could provide more robust support for COVID-19 epidemic prevention and control.
Keywords: Chinese visceral adiposity index, immune function, chronic inflammation, SARS-CoV-2 vaccine, neutralizing antibody
Introduction
The coronavirus disease (COVID-19) outbreak has ended, but sporadic cases still exist around the world and the virus strain continues to mutate. As of March 24th, 2024, the coronavirus disease (COVID-19) caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has infected 775.13 million people and caused 7.04 million deaths worldwide.1 Chinese Center for Disease Control and Prevention indicated COVID-19 positive rate has risen from 3.3% (in the 5th week of 2024) to 14.3% (in the 9th week of 2024) in China.2 The risk of contracting COVID-19 is never far away, and continues to pose a threat to global public health and socio-economic development.
Vaccination can reduce the incidence rates, severe cases, and mortality rates, making it the most cost-effective measure for controlling the COVID-19 pandemic.3–8 The COVID-19 vaccine activates B cells to differentiate into plasma cells and produces neutralizing antibodies primarily targeting the Spike protein. This process blocks the Spike protein receptor-binding domain (RBD) binding to host cell’s angiotensin-converting enzyme 2 (ACE2), preventing the invasion of SARS-CoV-2 into cells.9,10 At the same time, activated helper T cells (CD4+) secrete cytokines, activated B lymphocytes and activated killer T cells (CD8+) play cytotoxic role. However, the production of antibodies after vaccination is affected by many factors such as genetic factors, environmental factors and one’s own state. Our preliminary research showed obesity and air pollution can inhibit neutralizing antibody expression against SARS-CoV-2.11,12 Studies had shown that obese patients exhibited a poor response to COVID-19 vaccines due to their systemic chronic low-grade inflammation.13–17 Traditional obesity assessment depending on body mass index (BMI) or waist circumference (WC) has been inadequate in reflecting fat distribution and accurately assessing visceral adiposity.18–20 Research indicates that visceral adipose tissue, instead of total body fat, has a more significant impact on the inflammatory processes.21,22 However, the influence of the Chinese Visceral Adiposity Index (CVAI) on neutralizing antibodies (NAb) against SARS-CoV-2 remains poorly understood.
CVAI, a recently developed index based on research conducted on the Chinese population, is a better indicator reflecting visceral adiposity content.23 Visceral adiposity can induce immune cell infiltration and aggregation, release inflammatory cytokines, lead to immune dysfunction, and drive chronic inflammation. In addition, the abundant presence of ACE2 receptors in adipose tissue, all make individuals more susceptible to COVID-19 infection and prone to developing severe illness.24 Visceral obesity has been regarded as one risk factor for severe COVID-19.25–29 An increase of 10 square centimeters in visceral obesity area is related to a 1.37 times higher or 1.32 times higher of ICU treatment or mechanical ventilation, accordingly30 For one-unit increase in visceral fat tissue area, the likelihood of requiring intensive care increases by 2.47 times.31 Visceral fat is related to COVID-19 complications, which may link to chronic low-grade inflammation, both of which have been determined as a crucial risk factors of severity COVID-19 infections.24 Overall, individuals with visceral obesity are more likely to experience severe COVID-19 infections, which may be prevented efficacy by vaccination. Therefore, it is particularly important for individuals with visceral obesity to receive vaccinations and to ensure the effectiveness of vaccination to protect against the severe consequences of the disease.
The cross-sectional study aims to explore the impact of visceral adiposity on plasma NAb expression after SARS-CoV-2 vaccination and the potential mechanism, which may guide for optimizing vaccination guidance in visceral obesity. Therefore, the relationships among CVAI, chronic subclinical inflammation, innate and adaptive immune system, and plasma NAb expression were investigated.
Materials and Methods
Study Participants
This cross-sectional study recruited 206 healthcare workers (aged 20 to 50 years-old) during April 22nd to 23rd, 2021. All participants had obtained two doses inactivated SARS-CoV-2 vaccination (Vero cell, SINOVAC, China) on February 8th and March 10th, 2021, continuously. When biological samples were obtained, all participants were free of inflammation, had not used antibiotics, and had no mentioned health issues. Information on general characteristics, outdoor activities, dietary habits, educational level, night shift frequency, and house-hold socioeconomic status was gathered through a questionnaire survey on the SOJMP network platform. Before participation, all participants signed informed consent. This study was approved by the Medical Research Ethics Committee of Suining Central Hospital, China (LLSNCH20210012), and all procedures were complied with the principles of Declaration of Helsinki.
Clinical Laboratory Measurements
Trained nurses performed general physical examinations and collected the fasting venous blood samples. For EDTA anticoagulant blood samples (2 mL/tube): peripheral blood inflammatory cells (including white blood cells (WBC), neutrophils, monocytes, and lymphocyte counts) were analyzed by the XN-9000 automated hematology system (Sysmex, Japan), and the FACS Aria II flow cytometer (BD Bioscience, USA) was used to analyze the counts of peripheral blood natural immune cells, including B lymphocytes (CD3−CD19+), NK-lymphocytes (CD3−CD16+CD56+), and T lymphocytes (CD3+) as well as CD4+ T-lymphocytes (CD3+CD4+) and CD8+ T-lymphocytes (CD3+CD8+). Monoclonal antibodies (CD19-APC, CD16+56-PE, CD3-FITC, CD4-PC7, and CD8-APC-Cy7) were supplied by Beijing Tongsheng Shidai Biotech, China. Data processing was performed applying FlowJo software (version 7.6.1; BD Bioscience, USA). For separation gel-coagulant blood samples (5 mL/tube): after separating the serum at room temperature (3500 rpm, 5 minutes), Beckman AU5800 biochemical analysis system (Beckman Coulter Life Sciences, USA) used to quantify high-sensitivity C-reactive protein (hsCRP), and Siemens Atellica IM1600 chemiluminescence immunoassay analyzer was performed to assay interleukin (IL)-6 levels (Siemens AG, Germany). For heparin sodium anticoagulant blood samples (5 mL/tube): after separating the plasma at room temperature (3500 rpm, 5 minutes), Maccura i 3000 automatic chemiluminescence immunoassay analyzer (Maccura, China) was conducted to detect NAb titers, and the automatic Hitachi LST 7600 biochemical analysis system (Hitachi, Japan) was applied to estimate the personal health status.
Statistical Analysis
CVAI was calculated according to the formula in the reference:23
Males: CVAI = −267.93 + 0.68 × age (years) + 0.03 × BMI (kg/m2) + 4.00 × WC (cm) + 22.00 × LgTG (mmol/L) − 16.32 × HDL-C (mmol/L)
Females: CVAI = −187.32 + 1.71 × age (years) + 4.32 × BMI (kg/m2) + 1.12 × WC (cm) + 39.76 × LgTG (mmol/L) − 11.66 × HDL-C (mmol/L).
Participants were categorized into a reference group (CVAI ≤ 40.26, n = 103) and a dysfunction group (CVAI > 40.26, n = 103) based on the CVAI median. According to plasma NAb titers’ quartile, four participant groups were divided: Q1 ≤ 8.27 AU/mL, 8.27 AU/mL < Q2 ≤ 12.94 AU/mL, 12.94 AU/mL < Q3 ≤ 26.64 AU/mL, and Q4 > 26.64 AU/mL. Data distribution characteristics were evaluated by the Kolmogorov–Smirnov test. Differences in NAb titers, natural immune cell counts, and inflammatory cytokine levels among two CVAI groups were tested applying independent-sample t-test or the Mann–Whitney U-test. Multiple correspondence analysis model was conducted to identify the distribution correlation among CVAI, gender, and NAb titers. Pearson correlation analysis was conducted to determine factors related to CVAI and NAb. Additionally, multivariate adjusted linear regression analysis was conducted to evaluate dose-response relationship between CVAI, systemic inflammation, and plasma NAb titers. The covariates were calculated in two models: model a was adjusted for daily outdoor time/sleeping time/cigarette smoking, daily cigarette smoking for family members, alcohol drinking, window’s opening frequency, distance of residence from road, night shift frequency, educational status, and household monthly income, while model b was adjusted for factors other than smoking in Model a. The SPSS 22.0 (IBM, USA) statistic soft was performed to data analysis, and the GraphPad Prism 8.0 (GraphPad, CA) soft was conducted to figures’ edition. Statistical significance was deemed as P < 0.05 in two-tail.
Results
General Characteristics of These Participant Healthcare Workers
The present cross-sectional study recruited 206 healthcare workers (Table S1). The ratio of male-to-female was 66/140, and the median age was 31.00 years. BMI mean-value was 21.90 kg/m2, and the median CVAI was 40.26 (IQR 18.40–88.30). The median daily outdoor and sleeping times were 2.00 hours and 7.00 hours, respectively. Most participants did not smoke (87.38%) or drink (71.84%). Approximately 49.51% participants lived more than 100 meters from the road. Most participants frequently opened windows (85.92%). Around 76.70% participants had got the bachelor’s degree or higher education. About 50.97% participants’ household monthly income was over 10,000 yuan. About laboratory indicators, the median values of AST, ALT, triglycerides, uric acid, and creatinine were 21.00 U/L, 14.00 U/L, 1.32 mmol/L, 313.00 μmol/L, and 59.00 μmol/L, respectively. While the mean values of total protein, albumin, total cholesterol, HDL-C, LDL-C, and urea were 79.50 g/L, 48.48 g/L, 4.68 mmol/L, 1.24 mmol/L, 2.50 mmol/L, and 5.29 mmol/L, respectively.
Plasma NAb Titers and The Multiple Correspondence Correlation Among CVAI, Gender, and NAb Titers
The median plasma NAb titer was 12.94 AU/mL, and 181 participants (87.86%) had NAb titers ≥ 6 AU/mL (recommended reference threshold of the manufacturer), indicating the vaccine efficacy was 87.86% in healthcare workers against SARS-CoV-2. Compared with the reference group, lower NAb titers were observed in CVAI dysfunction group (median: 15.57 AU/mL vs 11.40 AU/mL, P < 0.05) (Figure 1). Concomitantly, participants had a lower vaccine efficacy in CVAI dysfunction group (86/103) than in reference group (95/103) (83.50% vs 92.23%, P < 0.05). Multiple correspondence analysis revealed that: (1) Although the Cronbach’s alpha coefficients were 0.634 and 0.019, the inertia were 0.577 and 0.338 for two dimensions, accordingly. That meant 91.50% of all variables may be explained well by the two dimensions (Table S2); (2) Two-dimensional model showed that CVAI > 40.26 was closely related to low NAb titers (NAb ≤ 8.27 AU/mL); Conversely, CVAI ≤ 40.26 was associated with high NAb titers (Nab > 26.64 AU/mL). Additionally, males were correlated with (8.27 AU/mL< Nab ≤ 12.94 AU/mL), while females were correlated with (12.94 AU/mL < Nab ≤ 26.64 AU/mL) (Figure 2).
 |
Figure 1 Expression of plasma-neutralizing antibody. Abbreviation: NAb, neutralizing antibodies. Note: *P < 0.05. |
 |
Figure 2 Two-dimensional graphs for multiple correspondence analysis of gender, CVAI, and plasma NAb titers. Abbreviations: CVAI, Chinese visceral adiposity index; NAb, neutralizing antibodies. |
Distribution Characteristics of Innate Immune Cells and Inflammatory Cytokines
Group difference analysis showed a more peripheral monocyte count in CVAI dysfunction group than in CVAI reference group (mean: 0.47 × 109/L vs 0.42× 109/L, P < 0.01) (Figure 3). There were no statistical significance for group differences in peripheral counts of WBC, neutrophils, monocytes, lymphocytes, T-lymphocytes, CD4+ T-lymphocytes, CD8+ T-lymphocytes, and CD4+/CD8+ ratio, B-lymphocytes, and NK-lymphocytes (P > 0.05) (Figure 3). Although no significant group difference was identified in serum IL-6 levels (P > 0.05), serum hsCRP levels were higher in CVAI dysfunction group than reference group (median: 0.50 mg/L vs 0.30 mg/L, P < 0.001) (Figure 4).
 |
Figure 3 The counts of peripheral blood immune cells. Note: **P < 0.01. |
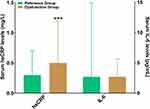 |
Figure 4 Serum concentrations of hsCRP and IL-6. Note: ***P < 0.001. |
The Relationships Among CVAI, NAb Titers, Innate Immune Cells, and Inflammatory Cytokines
Pearson correlation analysis suggested that CVAI was positively correlated with hsCRP (rs = 0.158), monocyte (rs = 0.212), lymphocyte (rs = 0.156), and B-lymphocyte (rs = 0.141) (all P < 0.05), whereas negatively related to NAb titers (rs = −0.143, P < 0.05) (Figure 5). Plasma NAb titers were positively associated with neutrophil and monocyte (rs = 0.176 and rs = 0.138, respectively, all P < 0.05), whereas negatively linked to lymphocyte, T-lymphocyte, and CD8+ T-lymphocyte (rs = −0.141, rs = −0.139, and rs = −0.153, respectively, all P < 0.05) (Figure 5).
Multivariable adjusted linear regression models were applied to further identify the dose‐respondent relationships of CVAI, NAb titers, inflammatory cytokines, and innate immune cells. Both rough models and adjusted models all showed the significant associations between increased CVAI and elevated levels of innate immune cell counts and inflammatory cytokines, as well as reduced NAb titers [B (95% CI) in adjusted models: 0.001 (0.000, 0.001) for monocyte, 0.003 (0.001, 0.005) for lymphocyte, 0.630 (0.152, 1.107) for B-lymphocyte, 0.007 (0.001, 0.013) for hsCRP, and −0.159 (−0.313, −0.005) for NAb titers, all P < 0.05] (Table 1). Results suggested with a unit increment in CVAI, the counts of monocyte, lymphocyte and B-lymphocyte, and hsCRP levels will increase by 0.001 (109/L), 0.003 (109/L), 0.630 (106/L), and 0.007 (mg/L), respectively, while the NAb titers will decrease on average with 0.159 AU/mL. Additionally, in the unadjusted models, elevated counts of neutrophil and monocyte were significantly associated with an increase in NAb titers [B (95% CI): 5.701 (1.305, 10.096) and 41.151 (0.411, 81.890), respectively, all P < 0.05] (Table 2). Decreased counts of lymphocyte, T-lymphocyte, and CD8+ T-lymphocyte were significantly associated with an increase in NAb titers [B (95% CI): −9.750 (−19.194, −0.307), −0.010 (−0.020, 0.000), and −0.021 (−0.040, −0.002), respectively, all P < 0.05] (Table 2). Further adjustment for daily outdoor time, daily sleeping time, daily cigarette smoking for family members, alcohol drinking, window’s opening frequency, distance of residence from road, night shift frequency, educational status, and monthly household income, the relationships of the counts of neutrophil, lymphocyte, and CD8+ T-lymphocyte with NAb titers remained significant [B (95% CI): 5.931 (1.437, 10.426), −10.373 (−20.196, −0.551), and −0.022 (−0.041, −0.003), respectively, all P < 0.05] (Table 2), which indicates that for every 1×109/L increase in neutrophils and lymphocytes, and every 1×106/L increase in CD8+ T-lymphocytes, the log transformation of NAb titers will increase by 5.931 units, and decrease by 10.373 units and 0.022 units, respectively.
 |
Table 1 The Dose–Effect Relationships of CVAI on NAb Titers, Inflammatory Cytokines and Innate Immune Cells |
 |
Table 2 Associations Among Innate Immune Cells and NAb Titers |
Discussion
This study found that compared with the reference group (CVAI ≤ 40.26), the dysfunction group (CVAI > 40.26) had an increase in peripheral monocyte counts, elevated hsCRP levels, and a decrease in NAb titers. Individuals’ CVAI was positively correlated with increased monocyte, lymphocyte, and B lymphocyte counts and hsCRP levels, but negatively correlated with NAb titers. This study shows that healthcare workers with visceral adiposity may potentially exhibit clustering of innate and adaptive immune cells, adopt an inflammatory phenotype, and drive local chronic low-grade inflammation, which inhibit plasma NAb expression to against SARS-CoV-2.
In clinical practice, levels of hsCRP and monocyte count in peripheral blood can serve as nonspecific markers of chronic inflammation.21, In this study, the hsCRP levels and peripheral monocyte counts of the CVAI dysfunction group were higher than reference group. Elevated CVAI was associated with increased peripheral hsCRP levels and monocyte counts. Previous research has shown a close association between excessive visceral adipose tissue and low-grade inflammation.21,22,31 Infection of visceral adipose cells by SARS-CoV-2 results in higher levels of pro-inflammatory markers compared to subcutaneous adipocytes.28 Therefore, our study further identified the relationship between high CVAI and the chronic inflammatory state in healthcare workers. Lymphocytes are crucial for immune response. T lymphocytes are primarily involved in cell-mediated immunity, and B lymphocytes were differentiated into plasma cells to generate antibodies, participating mainly in humoral immunity. In this study, there was a positive correlation between CVAI and the counts of lymphocyte and B lymphocyte. The counts of lymphocyte and CD8+ T-lymphocyte showed a negative correlation with NAb titers. Previous research has indicated that the excessive expansion of visceral adipose tissue, along with adipocyte hypertrophy and death, induces the infiltration and aggregation for immune cells (including macrophages, B lymphocytes, T lymphocytes, CD8+ T-lymphocytes, etc.), leading to the secretion of pro-inflammatory cytokines, driving chronic local inflammation.21 A positive feedback loop exists between local inflammation and changes in the immune response, ultimately resulting in systemic chronic low-grade inflammation.24,32 Our findings suggest that an increase in CVAI may lead to the infiltration and aggregation of lymphocytes, with a tendency towards T lymphocyte-dominant infiltration, thereby promoting the inflammatory state. While this study did not assess the primary immune-infiltrating cells (macrophages) in visceral fat, the significant increase in monocyte counts due to CVAI may favor their differentiation into macrophages, contributing to the onset and progression of inflammation. In summary, CVAI may lead to immune dysregulation and a state of chronic inflammation.
The study revealed that NAb titers were lower in the CVAI dysfunction group than reference group. CVAI > 40.26 was linked to the lowest NAb titers (NAb ≤ 8.27 AU/mL), while CVAI ≤ 40.26 was related to the highest NAb titers (NAb > 26.64 AU/mL). The NAb titers were negatively correlated with CVAI. Previous research has indicated that obese patients exhibited poor responses to vaccines such as COVID-19, influenza, hepatitis B, and rabies vaccines due to their systemic chronic low-grade inflammation.13–17 Our study suggests that visceral adiposity may have induced immune cell infiltration, resulting in systemic chronic inflammation, thereby inhibiting the production of neutralizing antibodies. Furthermore, men were associated with (8.27 < NAb ≤ 12.94 AU/mL), while women were associated with (12.94 < NAb ≤ 26.64 AU/mL). This could be attributed to the predominance of subcutaneous fat in females, while males tend to have higher levels of visceral fat.33,34 Obesity can induce B cell damage resulting in immunosuppression, leading to unsatisfactory antibody response after vaccination. Our results indicated that CVAI is positively associated with B lymphocytes, and detection of plasma cell content is needed to further study whether CVAI affects the transformation of B cells into plasma cells by affecting immune function and inflammatory state, resulting in the reduction of neutralizing antibody titers.
In this study, the median NAb titer among healthcare workers was 12.94 AU/mL, with 87.86% efficacy for SARS-CoV-2 vaccine (inactivated vero cell, SINOVAC, China). The effectiveness of two doses of CoronaVac in preventing symptomatic COVID-19 was 65.9% in Chile, 65% in Indonesia, and 83.5% in Turkey.6–8 The origin of these discrepancies may be attributed to different ethnicities and study populations. Our study focused on a small sample of healthcare workers, but it demonstrated the high expression of plasma NAb against inactivated SARS-CoV-2 vaccination (Vero cell, SINOVAC, China). Our research demonstrated that reducing visceral fat through exercise and dietary management,35 or lowering visceral fat by using glucagon-like peptide-1 receptor agonists (GLP-1Ras) in diabetes patients,36 may enhance the protective effect of inactivated COVID-19 vaccines against individuals with CVAI imbalance. The booster dose of the mRNA COVID-19 vaccine has increased the antibody response in individuals with abdominal obesity,37 suggesting that further research could be conducted to investigate whether the administration of booster doses has a positive impact on individuals with CVAI imbalance. CoronaVac and BNT162b2 vaccination, the antibody titers of severely obese patients were significantly lower than those of individuals with normal weight, but the antibody levels were significantly higher in severely obese patients who obtained BNT162b2 vaccination than those who received CoronaVac vaccination.14 This suggested that further research is needed to investigate whether different vaccines have different efficacy in patients with high CVAI, in order to provide more precise vaccine recommendations for this vulnerable group of individuals who require vaccination against COVID-19 even more.
Conclusion
In the present cross-sectional study, we investigated the relationships among CVAI, innate immune cells, inflammatory cytokines, and plasma NAb titers after SARS-CoV-2 vaccination. The results indicated that individuals with high CVAI have elevated levels of hsCRP and peripheral monocyte counts, concomitantly, plasma NAb titers are decreased. CVAI is positively correlated with hsCRP levels, peripheral monocytes, lymphocytes, and B cell counts, while negatively correlated with neutralizing antibodies. Overall, our study findings supported the hypothesis that visceral adiposity may induce immune cell infiltration and chronic inflammation, leading to impaired immune function and inhibition of neutralizing antibody expression. Thus, to enhance vaccine effectiveness and achieve herd immunity to combat the global COVID-19 pandemic, more precise vaccination strategies should be provided for high CVAI individuals who are in greater need of COVID-19 vaccination.
Abbreviations
CVAI, Chinese visceral adiposity index; NAb, neutralizing antibodies; WC, waist circumference.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
We acknowledge all the recruited healthcare workers for participating in this project.
Funding
This work was supported by the Natural Science Foundation of Sichuan Province, China (2023NSFSC1480) and the Talent Introduction Research foundation of the Second Affiliated Hospital of Chengdu Medical College (Nuclear Industry 416 Hospital), China (SYL2024RCYJ003).
Disclosure
The authors report no conflicts of interest in this work.
References
1. WHO. Coronavirus (COVID-19) Dashboard. Available from: https://covid19.who.int.
2. Chinese Center for Disease Control and Prevention (China CDC). National situation of novel coronavirus infection. Available from: https://www.chinacdc.cn.
3. Xu H, Li H, You H, et al. Effectiveness of inactivated COVID-19 vaccines against mild disease, pneumonia, and severe disease among persons infected with SARS-CoV-2 Omicron variant: real-world study in Jilin Province, China. Emerg Microbes Infect. 2023;12(1):2149935. doi:10.1080/22221751.2022.2149935
4. Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211.
5. Bates TA, McBride SK, Leier HC, et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci Immunol. 2022;7(68):eabn8014. doi:10.1126/sciimmunol.abn8014
6. Creech CB, Walker SC, Samuels RJ. SARS-CoV-2 Vaccines. JAMA. 2021;325(13):1318–1320. doi:10.1001/jama.2021.3199
7. Jara A, Undurraga EA, González C, et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N Engl J Med. 2021;385(10):875–884. doi:10.1056/NEJMoa2107715
8. Tanriover MD, Doğanay HL, Akova M, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, Phase 3 trial in Turkey. Lancet. 2021;398(10296):213–222. doi:10.1016/S0140-6736(21)01429-X
9. Yan R, Zhang Y, Li Y, et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi:10.1126/science.abb2762
10. Wang Y, Liu C, Zhang C, et al. Structural basis for SARS-CoV-2 Delta variant recognition of ACE2 receptor and broadly neutralizing antibodies. Nat Commun. 2022;13(1):871. doi:10.1038/s41467-022-28528-w
11. Zhang S, Chen S, Xiao G, et al. The associations between air pollutant exposure and neutralizing antibody titers of an inactivated SARS-CoV-2 vaccine. Environ Sci Pollut Res Int. 2022;29(9):13720–13728.
12. Hu J, Zhao M, Zhao Y, et al. Increased body mass index linked to decreased neutralizing antibody titers of inactivated SARS-CoV-2 vaccine in healthcare workers. Obes Sci Pract. 2022;9(1):23–29. doi:10.1002/osp4.626
13. Ledford H. How obesity could create problems for a COVID vaccine. Nature. 2020;586(7830):488–489. doi:10.1038/d41586-020-02946-6
14. Kara Z, Akçin R, Demir AN, et al. Antibody Response to SARS-CoV-2 Vaccines in People with Severe Obesity. Obes Surg. 2022;32(9):2987–2993. doi:10.1007/s11695-022-06181-y
15. O’Meara TR, Nanishi E, McGrath ME, et al. Reduced SARS-CoV-2 mRNA vaccine immunogenicity and protection in mice with diet-induced obesity and insulin resistance. J Allergy Clin Immunol. 2023;152(5):1107–1120. doi:10.1016/j.jaci.2023.06.031
16. Nasr MC, Geerling E, Pinto AK. Impact of Obesity on Vaccination to SARS-CoV-2. Front Endocrinol. 2022;13:898810. doi:10.3389/fendo.2022.898810
17. Watanabe M, Balena A, Tuccinardi D, et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab Res Rev. 2022;38(1):e3465. doi:10.1002/dmrr.3465
18. Zhang H, Zhan Q, Dong F, et al. Associations of Chinese visceral adiposity index and new-onset stroke in middle-aged and older Chinese adults: an observational study. Lipids Health Dis. 2023;22(1):74. doi:10.1186/s12944-023-01843-x
19. Lin M, Wu S, Deng X, et al. Visceral fat and its dynamic change are associated with renal damage: evidence from two cohorts. Clin Exp Hypertens. 2023;45(1):2271187. doi:10.1080/10641963.2023.2271187
20. Li B, Wang J, Zhou X, et al. Chinese visceral adiposity index is more closely associated with hypertension and prehypertension than traditional adiposity indices in Chinese population: results from the reaction study. Front Endocrinol. 2022;13:921997. doi:10.3389/fendo.2022.921997
21. Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–1832. doi:10.1038/s41591-019-0675-0
22. Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol. 2021;320(3):C375–C391.
23. Xia MF, Chen Y, Lin HD, et al. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult Chinese. Sci Rep. 2016;6:38214. doi:10.1038/srep38214
24. Colleluori G, Graciotti L, Pesaresi M, et al. Visceral fat inflammation and fat embolism are associated with lung’s lipidic hyaline membranes in subjects with COVID-19. Int J Obes. 2022;46(5):1009–1017. doi:10.1038/s41366-022-01071-w
25. Favre G, Legueult K, Pradier C, et al. Visceral fat is associated to the severity of COVID-19. Metabolism. 2021;115:154440. doi:10.1016/j.metabol.2020.154440
26. Yılmaz EM, Sehmen E, Arslan HN, et al. The effect of the visceral adiposity index on the severity of COVID-19 disease: results of a cross-sectional study. Eur Rev Med Pharmacol Sci. 2023;27(19):9446–9453. doi:10.26355/eurrev_202310_33973
27. Stefan N, Birkenfeld AL, Schulze MB. Global pandemics interconnected - obesity, impaired metabolic health and COVID-19. Nat Rev Endocrinol. 2021;17(3):135–149. doi:10.1038/s41574-020-00462-1
28. Saccon TD, Mousovich-Neto F, Ludwig RG, et al. SARS-CoV-2 infects adipose tissue in a fat depot- and viral lineage-dependent manner[J]. Nat Commun. 2022;13(1):5722. doi:10.1038/s41467-022-33218-8
29. Földi M, Farkas N, Kiss S, et al. Visceral Adiposity Elevates the Risk of Critical Condition in COVID-19: a Systematic Review and Meta-Analysis. Obesity. 2021;29(3):521–528. doi:10.1002/oby.23096
30. Petersen, A, Bressem, K, Albrecht, J, et al. The role of visceral adiposity in the severity of COVID-19: Highlights from a unicenter cross-sectional pilot study in Germany. Metabolism. 2020;110:154317. doi:10.1016/j.metabol.2020.154317
31. Watanabe, M, Caruso, D, Tuccinardi, D, et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism. 2020;111:154319 doi:10.1016/j.metabol.2020.154319.
32. Khan, S, Chan, YT, Revelo, XS, et al. The Immune Landscape of Visceral Adipose Tissue During Obesity and Aging. Front Endocrinol. 2020;11():267 doi:10.3389/fendo.2020.00267.
33. Bi, H, Zhang, Y, Qin, P, et al. Association of Chinese Visceral Adiposity Index and Its Dynamic Change With Risk of Carotid Plaque in a Large Cohort in China. J Am Heart Assoc. 2022;11(1):e22633. doi:10.1161/JAHA.121.022633
34. Kaisinger, LR, Kentistou, KA, Stankovic, S, et al. Large-scale exome sequence analysis identifies sex- and age-specific determinants of obesity. Cell Genom. 2023;3:8 100362. doi:10.1016/j.xgen.2023.100362
35. Winn NC, Cottam MA, Wasserman DH, et al. Exercise and Adipose Tissue Immunity: outrunning Inflammation. Obesity. 2021;29(5):790–801. doi:10.1002/oby.23147
36. Liao C, Liang X, Zhang X, et al. The effects of GLP-1 receptor agonists on visceral fat and liver ectopic fat in an adult population with or without diabetes and nonalcoholic fatty liver disease: a systematic review and meta-analysis. PLoS One. 2023;18(8):e289616.
37. Malavazos AE, Dubini C, Milani V, et al. BNT162b2 Booster Dose Elicits a Robust Antibody Response in Subjects with Abdominal Obesity and Previous SARS-CoV-2 Infection. Vaccines. 2023;11(12):1796. doi:10.3390/vaccines11121796
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


