Back to Journals » Clinical Ophthalmology » Volume 18
Visual Outcomes and Patient Satisfaction with a Hydrophobic Acrylic Monofocal IOL Delivered Using a Manual System
Authors Christenbury J, Hall B
Received 15 August 2024
Accepted for publication 13 November 2024
Published 26 November 2024 Volume 2024:18 Pages 3485—3491
DOI https://doi.org/10.2147/OPTH.S491589
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Joseph Christenbury,1 Brad Hall2
1Eye Consultants of Atlanta, Atlanta, GA, USA; 2Sengi, Penniac, NB, Canada
Correspondence: Joseph Christenbury, Eye Consultants of Atlanta, Atlanta, GA, USA, Tel +1-404-351-2220, Email [email protected]
Purpose: To evaluate visual outcomes and patient satisfaction at far and intermediate distances, up to 3 months postoperatively, in patients implanted with a hydrophobic acrylic intraocular lens (IOL) using a manual delivery system.
Methods: This was a prospective single-arm study of visual outcomes and patient satisfaction after successful bilateral cataract surgery with the Clareon monofocal and monofocal toric IOL using the Monarch IV delivery system. Endpoints included bilateral visual acuity, manifest refraction, and satisfaction assessed at 1 and 3 months post-operatively, as well as a surgeon survey of in-the-bag placement and satisfaction.
Results: A total of 28 patients (56 eyes) completed the study. At 3 months postoperatively, mean uncorrected and corrected binocular visual acuities at distance were 0.03 ± 0.04 logMAR and 0.00 ± 0.02 logMAR, respectively. For intermediate vision (66cm), 98% of eyes (55/56) had corrected monocular visual acuity 20/25 or better. In addition, mean uncorrected and corrected binocular visual acuities at intermediate (66cm) were 0.09 ± 0.05 logMAR and 0.04 ± 0.05 logMAR, respectively. Patient reported satisfaction with their vision was 88% (25/28). The IOL was delivered smoothly into the capsular bag in 89% (50/56) of eyes.
Conclusion: Clareon monofocal and monofocal toric IOL delivered with Monarch IV can provide excellent distance vision and intermediate vision, good spectacle independence, high reported patient satisfaction, consistent in-the-bag delivery, and high surgeon satisfaction.
Plain Language Summary: When the natural lens inside the eye becomes opaque (develops a cataract) it can be removed and replaced with an artificial intraocular lens (IOL). There are many different types of IOLs available for patients and surgeons to choose from. Monofocal IOLs are traditionally designed to provide clear vision at a single distance. However, recent advancements in optical designs for monofocal IOLs may provide functional vision when viewing objects at intermediate distances. The purpose of this study was to evaluate visual outcomes and patient satisfaction in patients with a new monofocal IOL, implanted with a manual delivery system, up to 3 months postoperatively. The results of this study suggest that the Clareon monofocal and monofocal toric IOL delivered with Monarch IV delivery system can provide excellent distance vision and functional intermediate vision.
Keywords: cataract surgery, monofocal, clareon, monarch IV
Introduction
The most often utilized intraocular lens (IOL) type during cataract surgery is the monofocal implant. Monofocal IOLs provide clear vision at a single distance, though traditionally patients may still require spectacles for good vision at intermediate and near distances. However, recent advancements in optical designs for monofocal IOLs, may provide functional intermediate vision with minimal impact to distance vision or increase in dysphotopsias.1,2
The Clareon monofocal IOL (Alcon Vision, LLC) is manufactured with a hydrophobic acrylic material. It is comprised of hydroxyethyl methacrylate (HEMA), phenylethyl acrylate, and a UV absorber. The water content has been reported to be 1.5% at 35°C.3 Previous studies have shown that the Clareon monofocal IOL (Alcon Vision, LLC) provides good visual outcomes for patients at distance with excellent optical clarity.4–7 There are also some reports of functional intermediate vision with this IOL.2,8
Advancements in IOL delivery systems may reduce surgical variability and improve procedural efficiency.9,10 The Monarch III manual delivery system was developed for delivering Alcon AcrySof IOLs. Studies have shown the Monarch III can consistently provide intracapsular bag delivery with a 2.49mm incision width.5 The Monarch IV manual delivery system was developed specifically for Clareon IOLs. Monarch IV has a precisely engineered plunger tip to provide unique grip of the Clareon IOL for consistent planar delivery. The Clareon monofocal (toric and non-toric) and the Monarch IV are US FDA approved, however clinical reports on the performance of Monarch IV are limited. In addition, there are no reports of patient satisfaction when using the Monarch IV and Clareon monofocal IOLs.
The purpose of this study is to evaluate visual outcomes and patient satisfaction at far and intermediate distances, up to 3 months postoperatively, in patients implanted with either Clareon monofocal or Clareon monofocal toric IOLs with the Monarch IV inserter. The results of this study could help patients—and surgeons—select the most appropriate IOL for their specific needs.
Methods
This was a prospective, single arm clinical evaluation of visual performance and patient satisfaction, after successful bilateral cataract surgery. As this study was conducted in private practice, an independent institutional review board (IRB) reviewed and approved the study (Salus IRB; approval JC-22-001). All subjects gave written informed consent before participation. The study followed International Harmonization (ICH) guidelines, Good Clinical Practice (GCP), and the tenets of the Declaration of Helsinki. The study was also registered in a clinical trial registry (clinicaltrials.gov; NCT05526781).
Adults (age 45 or older) with visually significant bilateral cataracts and no visually limiting conditions were included in the study. Exclusion criteria were visually significant co-morbidities (cornea, retina, optic nerve disease), history of neovascular AMD, diabetic macular edema, unstable proliferative diabetic retinopathy, geographic atrophy, branch retinal artery or branch retinal vein occlusion, central retinal artery or central retinal vein occlusion, moderate to severe epiretinal membrane, severe dry eye, history of intraocular infection, previous ocular surgery, history of rheumatoid arthritis, ocular cicatricial pemphigoid, Steven’s Johnson Syndrome, or history of glaucoma.
Preoperative biometry was measured with the IOLMaster 500 or IOLMaster 700 (Carl Zeiss Meditec AG), and IOL power was determined using the Barrett Universal II and Barrett Toric Calculator formulas. An A constant of 119.1 was used. Cataract extraction was performed by one experienced surgeon (JC) using phacoemulsification (stop and chop technique) and a clear 2.4 mm temporal corneal incision. The Monarch IV delivery system (Alcon Vision, LLC) was used to implant the Clareon monofocal or Clareon monofocal toric IOLs (CC60WF, CNA0T0, CNW0T3, CNW0T4, CNW0T5; Alcon Vision LLC). The postoperative regimen was the surgeon’s preferred standard of care.
Outcome measures were monocular and binocular uncorrected and corrected vision at distance (UDVA, CDVA), monocular and binocular uncorrected and distance-corrected vision at intermediate (66 cm; UIVA, DCIVA), manifest refraction, administration of a patient satisfaction questionnaire (IOLSAT), and administration of a surgeon questionnaire. The IOLSAT is a proprietary Alcon questionnaire that asks patients to rate their satisfaction and spectacle independence at various distances and under different lighting conditions (bright lighting conditions were 85 cd/m2 and dim lighting conditions were 3 cd/m2). The surgeon questionnaire asked about the consistency of the in-the-bag delivery of the IOL from the Monarch IV and surgeon satisfaction with the delivery of the IOL using the Monarch IV. Postoperative outcomes were measured at the 1 and 3 month visits. Visual acuities were collected in Snellen and converted to logMAR for analysis.11
This study was intended to be descriptive. Given that other studies of the Clareon monofocal IOL have included 30 to 50 subjects,2,5 it was anticipated that 35 subjects (70 eyes) would provide sufficient data to characterize the performance of the IOL and delivery system. The software R (version 4.4.0; The R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analysis.
Results
A total of 34 patients (68 eyes) completed bilateral IOL implantation. Of these, 5 were monovision patients that were excluded from the final analysis and one patient was lost to follow up, leaving a final dataset of 28 patients (56 eyes). Patient demographics and preoperative data are summarized in Table 1. No adverse events occurred related to the IOL or surgery.
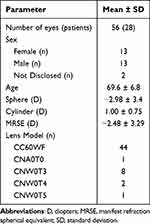 |
Table 1 Preoperative Data and Patient Demographics |
The primary outcome measures of this study were monocular and binocular UDVA and CDVA at 1 and 3 months postoperatively. These data are summarized in Figure 1 and Table 2. The percentage of eyes 20/20 or better for monocular UDVA and CDVA were 41% (23/56) and 96% (54/58) at 3 months postoperative, respectively, compared to 37% (21/56) and 89% (50/56) at 1 month postoperative, respectively. Similarly, the percentage of patients 20/20 or better for binocular UDVA and CDVA were 68% (19/28) and 96% (27/28) at 3 months postoperative, respectively, compared to 57% (16/28) and 93% (26/28) at 1 month postoperative, respectively.
 |
Table 2 Postoperative Visual Outcomes (logMAR) |
 |
Figure 1 Postoperative (3 month) uncorrected and distance corrected visual acuities for (A) Monocular Distance, (B) Binocular Distance, (C) Monocular Intermediate, and (D) Binocular Intermediate. |
Figure 1 and Table 2 also summarize monocular and binocular UIVA and DCIVA at 1 and 3 months postoperatively. The percentage of eyes 20/25 or better for monocular UIVA and DCIVA were 77% (43/56) and 98% (55/56) at 3 months postoperative, respectively, compared to 63% (35/56) and 93% (52/56) at 1 month postoperative, respectively. Similarly, the percentage of patients 20/25 or better for binocular UIVA and DCIVA were 93% (26/28) and 100% (28/28) at 3 months postoperative, respectively, compared to 79% (22/28) and 96% (27/28) at 1 month postoperative, respectively.
Table 3 summarizes the percentage of subjects that reported spectacle independence (selecting “Never” or “Rarely” on the IOLSAT questionnaire) at distance and intermediate under different lighting conditions. Overall, patient reported spectacle independence was substantially improved postoperatively at distance and intermediate compared to preoperatively. Reported spectacle independence was also generally higher at 1 month postoperative, compared to 3 months postoperative.
 |
Table 3 IOLSAT Questionnaire About Spectacle Independence |
Table 4 summarizes the percentage of subjects that reported “Good” or “Very Good” visual performance (on the IOLSAT questionnaire) at distance and intermediate under different lighting conditions. Overall, patient reported visual performance was greatly improved postoperatively at distance and intermediate compared to preoperatively. Reported visual performance at intermediate under dim conditions was higher at 1 month postoperative, compared to 3 months postoperative. In addition, the percentage of patients reporting satisfaction with their vision (“Satisfied” or “Very Satisfied” on the IOLSAT questionnaire) was 4% (1/28) preoperatively, 91% (26/28) at 1 month postoperatively, and 90% (25/28) at 3 months postoperatively.
 |
Table 4 IOLSAT Questionnaire About Visual Performance |
Other endpoints included refractive outcomes and a surgeon questionnaire. The percentage of eyes with ≤ 0.5 D of residual astigmatism and MRSE within ± 0.5 D were 91% (51/56) and 91% (51/56) respectively. Table 5 summarizes the responses to the surgeon questionnaire. Surgeon overall satisfaction was high, with the surgeon “Satisfied” or “Very Satisfied” in 100% (56/56) of eyes. Surgeon reported in-the-bag delivery was also high, with the surgeon reporting “IOL from cartridge coming out as planar, able to deliver IOL easily and smoothly into capsular bag” in 89% (50/56) of eyes. Of the 6 eyes where the surgeon indicated that the IOL was not delivered smoothly, reasons given were: the haptic was stuck on the optic (1/6 eyes), the trailing haptic did not unfold easily (1/6 eyes), and no reason was given (4/6 eyes).
 |
Table 5 Surgeon Questionnaire |
Discussion
This study evaluated the clinical outcomes, patient satisfaction, consistency of in-the-bag delivery, and surgeon satisfaction with the Clareon monofocal and monofocal toric paired with the Monarch IV manual delivery system. To the best of our knowledge, this is the first report on using the Monarch IV injector with the Clareon monofocal IOL. The IOL delivered with the Monarch IV came out as planar, and was able to be delivered easily and smoothly into the capsular bag in 89% of cases, according to the surgeon questionnaire. We are not aware of any other reports of surgeon experience with the Monarch IV. For context, several studies have reported high IOL controllability using an automated preloaded injector system (AutonoMe, Alcon Vision LLC) with the Clareon monofocal IOL.12–14 However, different delivery systems and questionnaires make comparisons difficult between studies.
In a large multicenter study, Lehmann et al15 reported on the outcomes with the Clareon IOL and Monarch III manual delivery system. In their study, mean monocular UDVA and CDVA were 0.04 and −0.05 logMAR, respectively, at 12 months postoperatively. This is similar to our results of mean monocular UDVA and CDVA of 0.06 and 0.00 logMAR, respectively, at 3 months postoperatively. Lehmann et al15 also observed that 85% of eyes had MRSE ≤0.50D, which is again similar to our study (91% of eyes had MRSE ≤0.50D). Other studies with the Clareon monofocal have reported similar distance visual acuity and refractive results.2,5,7,8,12
Interest for clear vision at intermediate distances is increasing, as many daily and important patient tasks are performed at this distance, such as viewing digital devices.16 Mean binocular UIVA and DCIVA (66 cm) at 3 months postoperatively were 0.09 and 0.04 logMAR respectively. This is a surprising result, given that the optic design of the Clareon monofocal is not modified to target intermediate vision. The range of intermediate visual acuities reported for enhanced-monofocal IOLs by a recent meta-analysis1 were 0.03 to 0.17 logMAR for UIVA17,18 and 0.01 to 0.14 logMAR for DCIVA.17,19 Our results would fall within these ranges. However, they differ from other published studies of the Clareon monofocal IOL. Blehm and Hall2 reported that mean binocular UIVA and DCIVA at 3 months postoperatively were 0.24 and 0.16 logMAR, respectively. Micheletti et al8 observed that mean binocular DCIVA at 3 months (or greater) postoperatively was 0.24 logMAR. Differences between our studies and other published studies may be due to patient populations, surgical technique, or other factors.
Spectacle independence at both distance and intermediate can improve patient quality of life.20 Patients have increasingly high expectations for spectacle independence, therefore, it is an important metric for understanding the success of IOL implantation. The high binocular visual acuities at distance and intermediate in our study translated to high patient reported spectacle independence at these distances. We are not aware of any other studies that have measured spectacle independence with the Clareon monofocal IOL. However, the AcrySof monofocal was used as a control in a recent US Food and Drug Administration trial.21 In this trial, spectacle independence with the monofocal at distance and intermediate under bright light conditions were 92% and 63%, respectively, and 78% and 51% under dim light conditions, respectively. These results are similar to our results with the Clareon monofocal (distance and intermediate under bright light conditions were 85% and 73%, respectively, and 77% and 54% under dim light conditions, respectively).
This study was intended to be a single arm descriptive study, which we acknowledge is a limitation. We are thus not able to draw conclusions about how the Monarch IV compares to other delivery systems, such as automated preloaded delivery systems. The relatively short follow up period is another limitation of this study. However, 3 months postoperatively is a typical of this type of study. Finally, this was a single surgeon study which may limit applicability to other surgeons.
In conclusion, the results of this study suggest that the Clareon monofocal and monofocal toric IOL delivered with Monarch IV can provide excellent distance vision and functional intermediate vision. Good spectacle independence can also be achieved at distance and intermediate. Patients reported high satisfaction with their vision at distance and intermediate. The Monarch IV had consistent in-the-bag delivery and high surgeon satisfaction.
Data Sharing Statement
Data are not available for sharing.
Acknowledgments
This paper was presented at the 2024 American Society of Cataract and Refractive Surgery (ASCRS) Annual Meeting as a poster.
Funding
This study was supported with an investigator-initiated study grant (73299167) from Alcon Vision LLC, Fort Worth, TX, USA.
Disclosure
Joseph Christenbury is a consultant for BioTissue and also receives honoraria from Dompe and Ocuphire. He also has received research grant support from Dompe. The authors report no other conflict of interest for this work.
References
1. Wan KH, Au ACK, Kua WN, et al. Enhanced monofocal versus conventional monofocal intraocular lens in cataract surgery: a meta-analysis. J Refract Surg. 2022;38:538–546.
2. Blehm C, Hall B. Evaluation of visual outcomes and 3-month refractive stability of a new hydrophobic acrylic intraocular lens. Clin Ophthalmol. 2023;17:1859–1864.
3. Maxwell A, Suryakumar R. Long-term effectiveness and safety of a three-piece acrylic hydrophobic intraocular lens modified with hydroxyethyl-methacrylate: an open-label, 3-year follow-up study. Clin Ophthalmol. 2018;12:2031–2037.
4. Oshika T, Fujita Y, Inamura M, Miyata K. Mid-term and long-term clinical assessments of a new 1-piece hydrophobic acrylic IOL with hydroxyethyl methacrylate. J Cataract Refract Surg. 2020;46:682–687.
5. Negishi K, Masui S, Torii H, Nishi Y, Tsubota K. Refractive stability of a new single-piece hydrophobic acrylic intraocular lens and corneal wound repair after implantation using a new automated intraocular lens delivery system. PLoS One. 2020;15:e0238366.
6. Werner L, Thatthamla I, Ong M, et al. Evaluation of clarity characteristics in a new hydrophobic acrylic IOL in comparison to commercially available IOLs. J Cataract Refract Surg. 2019;45:1490–1497.
7. Nuijts R, Bhatt U, Nanavaty MA, Roberts TV, Peterson R, Teus MA. Three-year multinational clinical study on an aspheric hydrophobic acrylic intraocular lens. J Cataract Refract Surg. 2023;49:672–678.
8. Micheletti JM, Duncan NB, Hall B. Head-to-head comparison of intermediate vision of two monofocal intraocular lenses. Clin Ophthalmol. 2023;17:3983–3990.
9. Mendicute J, Bascaran L, Pablo L, et al. Multicenter evaluation of time, operational, and economic efficiencies of a new preloaded intraocular lens delivery system versus manual intraocular lens delivery. Clin Ophthalmol. 2021;15:591–599.
10. Shimizu K, Kobayashi K, Takayama S, Zhaobin G. Preloaded injector for intraocular lens implantation without the use of ophthalmic viscosurgical devices. J Cataract Refract Surg. 2008;34:1157–1160.
11. Tiew S, Lim C, Sivagnanasithiyar T. Using an excel spreadsheet to convert Snellen visual acuity to LogMAR visual acuity. Eye. 2020;34:2148–2149.
12. Oshika T, Sasaki N, Clinical Study Group on New Intraocular L, Delivery S. One-year multicenter evaluation of a new hydrophobic acrylic intraocular lens with hydroxyethyl methacrylate in an automated preloaded delivery system. J Cataract Refract Surg. 2022;48:275–279.
13. Kim HK, Seo KY, Yoon KC, et al. Clinical Evaluation of a Hydrophobic Intraocular Lens Using a Preloaded Automated Injector in a Korean Population. Clin Ophthalmol. 2023;17:3353–3363.
14. Titiyal JS, Basak SK, Shetty N, et al. Twelve-Months Follow-Up Postmarket Study of a Hydrophobic Intraocular Lens Using a Preloaded Automated Injector in an Indian Population. Clin Ophthalmol. 2022;16:4215–4225.
15. Lehmann R, Maxwell A, Lubeck DM, Fong R, Walters TR, Fakadej A. Effectiveness and safety of the Clareon monofocal intraocular lens: outcomes from a 12-month single-arm clinical study in a large sample. Clin Ophthalmol. 2021;15:1647–1657.
16. MacRae S, Holladay JT, Glasser A, et al. Special report: American Academy of Ophthalmology task force consensus statement for extended depth of focus intraocular lenses. Ophthalmology. 2017;124:139–141.
17. Huh J, Eom Y, Yang SK, Choi Y, Kim HM, Song JS. A comparison of clinical outcomes and optical performance between monofocal and new monofocal with enhanced intermediate function intraocular lenses: a case-control study. BMC Ophthalmol. 2021;21:365.
18. Lopes D, Loureiro T, Carreira R, et al. Comparative evaluation of visual outcomes after bilateral implantation of an advanced or conventional monofocal intraocular lens. Eur J Ophthalmol. 2022;32:229–234.
19. Nanavaty MA, Ashena Z, Gallagher S, Borkum S, Frattaroli P, Barbon E. Visual acuity, wavefront aberrations, and defocus curves with an enhanced monofocal and a monofocal intraocular lens: a prospective, randomized study. J Refract Surg. 2022;38:10–20.
20. Blancafort Alias S, Del Campo Carrasco Z, Salvador-Miras I, et al. Exploring vision-related quality of life: a qualitative study comparing patients’ experience of cataract surgery with a standard monofocal iol and an enhanced monofocal IOL. Clin Ophthalmol. 2022;16:1641–1652.
21. McCabe C, Berdahl J, Reiser H, et al. Clinical outcomes in a U.S. registration study of a new EDOF intraocular lens with a nondiffractive design. J Cataract Refract Surg. 2022;48:1297–1304.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

