Back to Journals » The Application of Clinical Genetics » Volume 18
A Systematic Review of SNPs Screening for Platinum-Related Pharmacodynamics and Pharmacokinetics Genes in Non-Small Cell Lung Cancer for Precision Medicine
Authors Afifah NN , Larasati AL , Wijaya I , Zakiyah N , Intania R, Obinata H, Barliana MI
Received 20 January 2025
Accepted for publication 28 May 2025
Published 27 June 2025 Volume 2025:18 Pages 93—112
DOI https://doi.org/10.2147/TACG.S518467
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Martin Maurer
Nadiya Nurul Afifah,1,2 Annisa Lazuardi Larasati,3 Indra Wijaya,4 Neily Zakiyah,5,6 Ruri Intania,7 Hideru Obinata,8 Melisa Intan Barliana1,6
1Department of Biological Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, Indonesia; 2Department of Pharmacy, Faculty of Health Sciences, Universitas Esa Unggul, Jakarta, Indonesia; 3Faculty of Pharmacy, Universitas Indonesia, Depok, Indonesia; 4Department of Internal Medicine, Hasan Sadikin General Hospital, Bandung, Indonesia; 5Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, Indonesia; 6Center of Excellence for Pharmaceutical Care Innovation, Universitas Padjadjaran, Sumedang, Indonesia; 7Rotinsulu Lung Hospital, Bandung, Indonesia; 8Department of Biochemistry, Gunma University School of Medicine, Maebashi, Japan
Correspondence: Melisa Intan Barliana, Email [email protected]
Introduction: Traditional treatments for non-small cell lung cancer (NSCLC), such as chemotherapy, especially platinum-based regimens, often lack efficacy due to the disease’s inherent heterogeneity. Precision medicine in NSCLC recognizes each tumor’s unique genetic profile. Alterations in the pharmacokinetics and pharmacodynamics of platinum-based therapies significantly influence their clinical outcomes. Previous research has predominantly focused on genetic polymorphisms in genes like Glutathione S-transferase Pi 1 (GSTP1), ATP Binding Cassette subfamily C member 2 (ABCC2), Excision repair cross-complementation group 1 and 2 (ERCC1, and/ ERCC2), which play crucial roles in detoxification, drug transportation, and Nucleotide Excision Repair (NER). However, findings have shown considerable variability.
Methods: The analysis followed the PRISMA and STROPS Guidelines, using specific search terms including NSCLC, Chemotherapy, Polymorphisms, Single Nucleotide Polymorphisms (SNPs), ERCC1, ERCC2, ABCC2, GSTP1, Effectiveness, and Clinical Response. These studies were subjected to full-text screening process.
Results: Initial screening of 370 studies, comprising 275 from PubMed and 95 from EBSCO, identified 53 relevant ones, excluding those such as reviews, non-English studies, and meta-analyses. Among the genetic variants studied (ERCC1 rs11615, ERCC2 rs13181, ABCC2 rs717620, GSTP1 rs1695), GSTP1 rs1695 emerged as particularly promising, with 11 studies indicating a significant association with improved survival outcomes.
Conclusion: The integration of SNP profiling into clinical decision-making processes holds substantial potential for enhancing the personalization of NSCLC treatment strategies, thereby improving patient outcomes.
Keywords: NSCLC, chemotherapy, polymorphisms, SNPs, ERCC1, ERCC2, ABCC2, GSTP1
Introduction
Precision medicine in non-small cell lung cancer (NSCLC) is increasingly becoming a pioneering method to revolutionize cancer treatment. The foundation for modern treatment approaches in NSCLC can be traced back to 1969, when cisplatin one of the first effective chemotherapy agents was discovered. Subsequently, targeted therapies and immunotherapies were developed, leading to the occurrence of the pharmacogenomics era, which aimed to maximize efficacy and reduce adverse effects.1,2 Traditional treatment, including chemotherapy, particularly platinum-based regimens, as well as modalities such as radiation and surgery, are often associated with limited success due to the inherent heterogeneity of the disease.3 However, recent advances in genomics and molecular biology have shown information regarding the genetic alterations influencing NSCLC and patient responses to chemotherapy.4 This information has been used for the development of specific therapies to target certain genetic mutations and predict responses to chemotherapy.5–8 However, accessibility and affordability of innovative treatments, including targeted therapy and immunotherapy, pose significant challenges, particularly in low- and middle-income countries (LMICs).9–11 Despite the accessibility of targeted and immunotherapy treatments, some therapies have shown minimal or no response in patients with NSCLC. In previous studies, the overall response rate has been limited when using only targeted therapy or immunotherapy, leading to the combination of biologic agents with conventional chemotherapy, such as paclitaxel and platinum-based regimens, to potentially improve treatment efficacy.12–14
Platinum-based chemotherapy as the first-line conventional treatment in NSCLC is widely used, particularly in patients with a wildtype or negative profile for biomarkers such as EGFR, PD-L1, ROS1, and ALK fusion.15 Studies focusing on personalized medicine in targeted therapy and immunotherapy related to clinical outcomes such as HR and PFS are more significant compared to others on cytotoxic or conventional chemotherapy. Previous meta-analyses had shown that there was no personalized method in clinical trials for conventional chemotherapy.16 Meanwhile, the effectiveness of platinum is varied based on genetic profiles, particularly gene markers in the pharmacokinetics and pharmacodynamics of platinum.17–21
The genetic profile, including polymorphism on Glutathione S-transferase Pi 1 (GSTP1) as detoxification enzymes and ATP Binding Cassette subfamily C member 2 (ABCC2) as the transporter, can influence the pharmacokinetics mechanism on platinum-based. Meanwhile, the polymorphism of Excision repair cross-complementation group 1 and 2 (ERCC1, and/ ERCC2) genes that play a role in Nucleotide Excision Repair (NER), is capable of influencing pharmacodynamics mechanism. This polymorphism can alter the expression level of protein and activity, leading to the clinical outcomes of chemotherapy.22–25 The identification of genetic aberrations also allows clinicians to optimize treatment outcomes, minimize adverse effects, and enhance the prognosis for NSCLC patients. Consequently, screening these genetic markers represent a novelty that can fundamentally impact NSCLC therapy, particularly for platinum-based conventional chemotherapy, offering more effective and less toxic treatment options.26,27
In recent years, several databases and tools have been developed to support the analysis and interpretation of single nucleotide polymorphisms (SNPs). One of the most widely used resources is ClinVar, a publicly accessible database that aggregates information about genomic variation and its relationship to human health. Another key resource is PharmGKB, which focuses on the impact of genetic variation on drug response and provides curated information related to pharmacogenomics. In addition to these databases, various computational tools such as SIFT, PolyPhen, and MutationTaster are commonly used to predict the functional impact of SNPs. These resources play a crucial role in SNP profiling by helping to prioritize variants for further investigation and guiding clinical interpretation.
Based on the background above, this systematic review aimed to identify, assess, and summarize the single nucleotide polymorphisms (SNPs) in key genes, namely ERCC1, ERCC2, GSTP1, and ABCC2, to determine their potential as predictive markers for therapy outcomes in NSCLC. The analysis focused on determining which SNPs could serve as a reliable genetic marker for platinum-based therapies. The results are expected to establish a framework for SNPs screening in NSCLC patients who tested negative for genetic alterations such as EGFR mutations, ALK fusion, ROS1 rearrangements, or PD-L1 expression. Additionally, the personalized method aimed to enhance decisions on treatment guidance and optimize therapeutic strategies for NSCLC patients.
Materials and Methods
Literature Search Strategy and Identification
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA)28 (Supplementary 1 and 2). Additionally, the reporting quality for all included studies was assessed using Checklist Guidelines for Pharmacogenomic Studies, STrengthening the Reporting of Pharmacogenetic Studies (STROPS).29 A systematic literature review was conducted from June to July 2023, with two authors responsible for collecting and analyzing the data. We selected studies that were eligible for inclusion using the STROPS Guidelines and predefined inclusion and exclusion criteria (Supplementary 3). Literature searches were performed using two databases, PubMed and EBSCO. The investigation was conducted to obtain relevant studies on SNPs in ERCC1, ERCC2, ABCC2, and GSTP1 genes, along with their correlation to the platinum-based chemotherapy responses, including survival. Search strategies included the use of the following terms, namely NSCLC, Chemotherapy, Polymorphisms, SNPs, ERCC1, ERCC2, ABCC2, and GSTP1, including Effectiveness, and Clinical Response (Supplementary 4). The selection of keywords referred to the population, intervention/exposure, and outcomes (PICO/PECO) method. The selected population consisted of patients with lung cancer, specifically NSCLC, and the observed exposure included ERCC1, ERCC2, ABCC2, and GSTP1 gene polymorphism. Meanwhile, the outcomes focused on the chemotherapy platinum-based responses such as Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1), survival rate, mortality rate, and prognosis.
Study/ Literature Selection
The screening process was carried out in two stages, title and abstract, followed by full text. This systematic review included all published studies that met the inclusion criteria without time restriction. The inclusion criteria used for the literature screening process comprised human studies with NSCLC, focusing on pharmacogenetic studies detailing genotypes and physiological effects, which are chemotherapy responses. However, the exclusion criteria were review studies, including non-English and non-human, along with short communication, editorial board, expert opinion, non-platinum therapy, adjuvant/neoadjuvant chemotherapy combined with a biologic agent (multi-modality), unrelated studies, and meta-analysis.
Data Extraction and Synthesis
Data were extracted on study characteristics including design, country, number of subjects, SNPs variant (gene and rs number), base changes, clinical manifestation, genotyping methods, statistical value, and author. To streamline data extraction, a spreadsheet was used for collecting the results, as shown in Table 1 and the summary was presented in Table 2. Initially, the identification of clinical manifestations was carried out to facilitate data synthesis The manifestations were based on the chemotherapy responses such as RECIST. 1.1, survival rate, mortality, prognosis, or treatment response rate discussed in all studies, as fully explained in tables and figures.
 |
Table 1 Polymorphism of ERCC1, ERCC2, ABCC2, and GSTP1 on NSCLC Clinical Outcomes |
 |
Table 2 Resume of Single Nucleotide Polymorphism and the Interpretation Related to Clinical Outcomes |
Results and Discussion
Systematic Search
Based on literature search strategy, identification, and selection process, 370 studies were obtained, consisting of 275 on PubMed and 95 from EBSCO. A total of 107 studies were collected after the title and abstract screening. At this stage, the exclusion criteria were applied, focusing on editorial, unrelated studies, review, meta-analysis, non-chemo-naive, multimodality, non-human, and duplicate between two databases. Subsequently, full-text screening was carried out, resulting inclusion of 52 studies, as shown in Figure 1. After data extraction and analysis of the result, a total of 14 variants of SNPs were obtained, including ERCC1 (rs3212986, rs11615, rs2298881), ERCC2 (rs13181, rs1052555, rs238406, rs1799793), ABCC2 (rs717620, rs2273697, rs3740066), and GSTP1 (rs1695, rs1138272, rs3740066).
 |
Figure 1 Research Flowchart. |
Main Findings
The use of comprehensive keywords, such as “non-small cell lung cancer (NSCLC)” and “chemotherapy”, is essential to identify the specific population. Additionally, the inclusion of terms such as “polymorphisms” and the genes “ERCC1”, “ERCC2”, “ABCC2”, and “GSTP1” is crucial for determining the exposure factors. The STROPS checklist guideline is applied to ensure that the included studies meet the standards expected for reporting in the field of pharmacogenetics (Online Resource 3). Among the studies included, a significant portion of pharmacogenetics studies failed to provide explanations for addressing issues such as false positive results, multiple genetic variants, several outcomes, and various assumptions regarding the mode of inheritance.30,32,50,51,64,68 Additionally, the majority did not sufficiently address missing data were handled.30–32,50,51,64,68 Another checklist point on STROPS is related to the rs number of polymorphisms, where approximately half of the studies provided the rs number for each genotyped SNP. Regarding polymorphisms, the majority of the studies included used alternative names such as C3972T, C-24T, or G1249A.64–66 Consequently, this systematic review conducted data extraction and summarized results using the rs numbers for individual polymorphisms.
The main objective is to investigate the impact of genetic variations on chemotherapy responses, with potential outcome measures including survival rates, mortality, treatment, and RECIST 1.1 criteria. During the data extraction process, various clinical manifestations were observed, which influenced the results. Polymorphisms in ERCC1 in 12 studies were associated with decreased or increased chemotherapy responses and survival rates.30,32,34–37 Meanwhile, 15 studies reported non-statistically significant associations with chemotherapy responses and survival rates.30–33,36 The majority of polymorphisms in ERCC2, comprising 18 studies, did not show statistically significant correlations with platinum-based chemotherapy responses.44,48,53,59,61–63 Mutant alleles of ABCC2 and GSTP1 polymorphisms were found to potentially enhance chemotherapy responses and improve survival rate,33,65–68 as shown in Table 1. This suggested that SNPs variants could have a significant impact on protein structure, potentially leading to the upregulation or downregulation of protein formation or altering gene expression levels.78–82 The results showed promise as a marker for personalized medicine, particularly in early genetic screening for NSCLC cases before initiating platinum-based therapy.
Implications of These SNPs for NSCLC Clinical Response
Cancer is the leading cause of mortality rate globally, accounting for approximately 10 million deaths in 2020.83 Ranking as the first cancer-related fatalities, the issue of lung cancer is related to the high incidence of new cases with low survival or elevated mortality rates. Moreover, survival rates have shown a significant decreasing trend, ranging from 94% to 91% and 78%, from the first to the third year, respectively.84 The fifth-year relative survival rate for NSCLC as a whole remains extremely low at 24%.85 Platinum-based chemotherapy, as a first-line treatment used in NSCLC patients with wildtype profiles on EGFR gene, has been found to show varying responses. Previous studies have shown that the Objective Response Rate (ORR) for platinum-based first-line chemotherapy ranged from 29.7% to 46.7%, while others reported a significant proportion between 0% and 80%.86–88
The variability in responses among patients receiving platinum-based chemotherapy is attributed to factors such as the clinical status or the influence of genetic polymorphisms on genes in the pharmacodynamics or pharmacokinetics, as shown in Figure 2.
 |
Figure 2 Single Nucleotide Polymorphism Screening Implication on Non-Small Cell Lung Cancer. |
According to the CPIC database, ERCC1 has a D level of evidence for cisplatin, and GSTP1 also has a D level for several agents, including cyclophosphamide, oxaliplatin, epirubicin, and fluorouracil. Additionally, ABCC2 and GSTP1 are not listed in the CPIC gene-drug database. A D level of evidence means that there are few published studies, the clinical significance is unclear, the mechanistic basis is weak, or the data is conflicting. Since these genes are not commonly tested in clinical settings, further research is needed to strengthen the evidence base. This review discusses SNPs in ERCC1 and ERCC2 genes related to the pharmacodynamics of platinum-based chemotherapy, including SNPs in ABCC2 and GSTP1 genes related to the pharmacokinetics of platinum-based drugs. All of these genes show the potential to influence clinical outcomes, including Overall Survival (OS), Progression-Free Survival (PFS), the risk of mortality, and Time to Progression.24,30,32,36,63,64,89 Clinical manifestations, such as disease progression and the Risk of Metastasis, are specifically associated with ERCC1 and ERCC2 genes,30,50 and the risk and rate of toxicity are related to ABCC2 and GSTP1 genes66 as shown in Figure 2.
From the review results, we can see that studies on ERCC1 that produced significant statistics mostly had odds ratios (OR) and hazard ratios (HR) that were not very large (ranging from 0 to 1). Clinically, this suggests that the impact of these SNPs on clinical outcomes is likely not substantial, although one study reported an OR of 10.161 with a p-value of 0.001.35 A similar situation is observed with ERCC2, while GSTP1 and ABCC2 had larger OR and HR values. However, the clinical relevance of these numbers also depends on factors such as sample size, study design, and biological plausibility.
In addition, it is important to acknowledge that ethnic variation can significantly influence the frequency and clinical impact of pharmacogenetic polymorphisms. For example, according to the PharmGKB and gnomAD databases, allele frequencies for several SNPs in ERCC1, ERCC2, ABCC2, and GSTP1 differ across populations. The ERCC1 rs11615 C allele, for instance, has been reported to be more prevalent in East Asian populations compared to Europeans or Africans, which may affect its predictive value for platinum response across different ethnic groups.90,91 However, in our systematic review, stratified analyses based on ethnicity were not feasible due to inconsistent reporting of ethnic background and limited population-specific data. Nonetheless, we recognize that functional consequences of SNPs are not solely determined by allele frequency, but also by the nature of the nucleotide substitution, which can lead to changes in codon usage, amino acid sequence, or protein structure. These considerations underscore the importance of ethnicity-aware pharmacogenomic research and the need for further population-specific studies before SNP-guided therapy can be generalized to diverse clinical settings.
Molecular Mechanisms of ERCC1, ERCC2, ABCC2, and GSTP1 SNPs to Clinical Outcomes in NSCLC
ERCC1 and ERCC2 genes encode proteins that play a significant role in the unwinding processes within the NER mechanism,92,93 as shown in Figure 3. Alterations in NER mechanism activity, caused by SNPs, can impact responses to platinum-based chemotherapy,93–95 serving as predictive markers for chemotherapy responses. This review compiled 31 studies discussing ERCC1, with 7 focusing on rs3212986, 20 on rs11615, and 4 on rs2298881. The results show that ERCC1 rs11615 has the largest number of studies, indicating prominence as a major focus due to relevance in clinical manifestations, as shown in Table 2. However, a total of 9 studies related to ERCC1 rs11615 were identified, which did not show statistically significant associations with platinum-based clinical outcomes. Among the 7 studies reviewed, the mutant allele of rs11616 was associated with decreased clinical outcomes and shorter survival in NSCLC patients treated with platinum-based therapy. In the molecular mechanism of ERCC1 rs11615 was found to start from the substitution of Cytosine (C) with Thymine (T), resulting in the modulation of ERCC1 expression levels. Meanwhile, in the ERCC1 C8092A (rs3212986), situated in the 3’-untranslated region (3’-UTR), the presence of the A allele on rs3212986 was associated with high ERCC1 expression, playing a significant role in transcription and translation processes.96–98 This alteration affected DNA repair processes, as high activity caused by the increase in ERCC1 expression level diminished the efficacy of platinum-based therapy.96
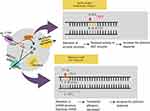 |
Figure 3 Single Nucleotide Excision Repair on ERCC1 and ERCC2. |
A total of 30 studies were compiled for ERCC2/ XPD, with 15 focusing on rs13181, 5 on rs1052555, 3 on rs238406, and 7 on rs1799793. The SNPs on ERCC2, specifically rs13181, have been extensively investigated, indicating a significant change from A to C, as shown in Table 2. This alteration leads to a change in the amino acid produced, shifting from Lysine (Lys) to Glutamine (Gln)99 as shown in Figure 3. However, 12 out of the 15 studies reviewed reported non-statistically significant results regarding the association between ERCC2 rs13181 and the clinical outcomes or survival rates of NSCLC patients receiving platinum-based chemotherapy. These results suggested that the specific SNPs are not essential predictors of treatment response or patient survival in platinum-based chemotherapy for NSCLC. Subsequently, ERCC2 rs1799793, which ranks second in terms of the number of ERCC2 genes studies, has shown a balanced outcome. The results are evenly distributed between studies reporting non-statistically and statistically significant association of the mutant allele with a decrease in responses to platinum-based chemotherapy. This suggests a complex relationship between ERCC2 rs1799793, affecting the clinical outcomes or treatment responses. The ERCC1 rs1799793 is characterized by a change from G to A and the resulting amino acid transitioned from Aspartic Acid (Asp) to Asparagine (Asn).58 Both rs13181 and rs1799793 are common non-synonymous SNPs, located within the ERCC2 coding sequence, with specific functions that impact the NER pathway by modulating mRNA expression.60,100 These changes in amino acids have the potential to modify protein expression levels and influence the capacity for DNA damage repair, which is a fundamental process in NER.22 Specifically, the enhanced functionality of NER associated with the mutations is correlated with reduced chemotherapy response.100
In a study conducted on a European population, a statistically significant trend was observed for the allele G → T on rs238406, which affected ERCC2 mRNA expression (Ptrend = 0.011). However, in the Chinese Han in Beijing (CHB) population, there was only a borderline significance (Ptrend = 0.098).101 Thus, phenomenon occurred due to the presence of linkage disequilibrium with rs13181 or other potentially functional SNPs. In this review, rs238406 was observed to be more closely associated with the risk of NSCLC, although there were no statistically significant correlations with chemotherapy response.50,53,54,102 The results provided valuable information on the intricate relationship between genetic variations in ERCC2 and their impact on the NER pathway, affecting the response to platinum-based chemotherapy.103
In pharmacokinetics, there are several essential processes, including absorption, distribution, metabolism, and elimination such as detoxification.104,105 Numerous genes have shown significant association with these mechanisms, where genetic polymorphisms cause alterations in protein expression, affecting the rate of pharmacokinetic processes such as metabolism (CYP genes), detoxification (GSTs genes), or drug concentration at target sites when genetic variants impact transporter proteins like ABC transporters.77,106,107 Among the essential genes, GSTP1 is responsible for encoding Glutathione enzymes,69,72,77,108–110 while ABCC2 is used for coding the ABC transporter protein.25,111–113 These genes play significant roles in pharmacokinetic process, influencing detoxification rates and facilitating the transport of active compounds such as platinum-based compounds, respectively.
GSTP1 is the primary Phase II detoxification enzyme predominantly located in the cytosol, which facilitates the bonding of electrophilic substances with glutathione (GSH), showing peroxidase and isomerase functions. This enzyme suppresses the activity of Jun N-terminal kinase, thereby protecting cells from death induced by hydrogen peroxide (H2O2). GSTP1 possesses the ability to non-catalytically bind to a diverse array of naturally occurring and external ligands.114 Furthermore, it plays a specific role in the detoxification process of platinum compound, which effectively captures and deactivates cisplatin, using two exposed cysteines. This phenomenon results in the interlinking of protein subunits, retaining the capability to perform GSH-conjugation activities.115 Genetic polymorphism at GSTP1 rs1695, situated on chromosome 11 in exon 5, induces an alteration in the amino acid produced, replacing isoleucine with valine (Ile105Val). This change includes a transition from A to G in the base pair, leading to the suppression of protein synthesis and decreased GSTP1 enzyme activity. As shown in Figure 4, the decrease in GST activity causes an increase in platinum-based chemotherapy responses.116,117 In this review, a total of 18 studies were investigated, focusing on the impact of GSTP1 polymorphisms, as shown in Table 2. Based on the results GSTP1 rs1695 was the most extensively investigated, with 15 studies dedicated to the exploration. Other variations such as rs1138271, rs147282497, and rs781659437 had only one study each. Furthermore, rs1695 was found to be statistically significant in 11 cases, indicating that the presence of the mutant allele (AG or GG genotype) was associated with increased platinum-based chemotherapy responses and enhanced survival, as shown in Table 2.
 |
Figure 4 Single Nucleotide Excision on GSTP1 and ABCC2. |
Regarding ABC transporters, particularly the ABCC2/MRP genes, a total of 10 studies were identified, where 4 focused on ABCC2 rs717620, 3 on rs2273697, and 3 on rs3740066, as shown in Table 2. Based on the results, ABCC2 rs717620 showed that the presence of the mutant allele was associated with improved chemotherapy responses and enhanced survival rates. However, in one remaining study, the mutant allele was related to a decrease in survival rates among NSCLC patients treated with platinum-based chemotherapy. At the molecular level, ABCC2 showed low expression in normal lung tissue but had high expression in lung cancer. The ABCC2 rs717620 polymorphism, situated within ABCC2 transcript promoter, 5’ UTR, included a –24C > T variant that could potentially reduce ABCC2 expression, particularly in tumour tissue. This phenomenon described the observed suppression of drug resistance and the enhancement of treatment responses112,118 as shown in Figure 4. However, the nonsynonymous rs2273697 (Val417Ile) and the silent rs3740066 (Ile1324Ile) polymorphisms did not show statistical significance in relation to chemotherapy responses.
Besides SNPs, Copy Number Variant (CNV) also influence clinical outcomes. When SNPs and CNVs occur at the same location, they can affect clinical outcomes.119,120 These involve larger sections of the genome being duplicated or deleted. CNVs can change the dosage of gene products (eg, more or fewer copies of a gene), which can also influence clinical traits, such as disease risk or drug metabolism.121 For instance, a study demonstrated that a deletion in the TBX6 gene led to an overestimation of the impact of SNPs on the hypomorphic allele. This study also generalized a model to explain calculation bias or distorted significance in association studies caused by CNVs at specific loci. Moreover, the overlap between disease-associated SNPs from published GWAS and common CNVs and pathogenic/likely pathogenic CNVs was significantly higher than random distribution, suggesting that co-occurrence of CNVs and SNPs at the same locus can significantly influence data interpretation and the potential outcomes of GWAS.119
In this study, several CNVs were observed at the same loci as important SNPs, including ERCC1 rs11615, ERCC2 rs13181, ABCC2 rs717620, and GSTP rs1695, and they exhibited molecular effects. For example, the NSV3163036 on ERCC1 impacts not only the coding sequence but also the 5’ UTR and intron regions, which could alter gene expression or splicing.122 Similarly, CNV on ERCC2 NSV2785402, GSTP1 (NSV555269, NSV468606), and ABCC2 NSV3155816 have the molecular effect on both the coding sequence regulatory regions, potentially modifying function.123–129 While the molecular impacts of these SNPs are well documented, their interplay with CNVs at the same loci may amplify or mitigate their effects. To fully understand how CNVs influence the functional impact of SNPs, further research using methods designed to assess these combined effects is necessary.
Role of SNP Screening in NSCLC Precision Medicine
Research and development related to cancer treatment have made significant advancements. This development was initially observed in 1969 when platinum-based chemotherapy, particularly cisplatin, showcased promising efficacy in treating NSCLC. In 1995, platinum-based therapy was discovered as a significant contributor to increased survival rates among NSCLC patients. Subsequently, in 2004, the introduction of Tyrosine Kinase Inhibitors (TKIs) as targeted therapies customized to EGFR mutation profiles offered an essential contribution, with drugs such as gefitinib, marked a significant milestone. Erlotinib also gained approval as a second-line therapy for EGFR mutation-positive cases. In 2015, advanced medications such as Crizotinib showed significant effectiveness in managing ROS1-rearranged NSCLC, enriching the therapeutic landscape for the disease. Currently, there is continuous advancement in medication development for NSCLC, guided by genetic profiling for personalized treatment methods.1 The standard for cancer treatment has shifted towards the use of biological agents such as targeted therapy and immunotherapy. However, platinum-based chemotherapy, a conventional cytotoxic agent, continues to be a widely used first-line treatment option Despite these numerous applications, the effectiveness and toxicity rates of platinum-based chemotherapy have shown significant variation. SNPs on several genes, particularly those that play essential roles in the pharmacokinetic or pharmacodynamic mechanisms of platinum-based chemotherapy, have become potential predictive markers for treatment responses. This has led to the application of SNPs screening to conventional chemotherapy regimens based on genetic profiles of patients. Consequently, patients who test negative for established biomarkers such as EGFR, PD-L1, ALK fusion, ROS-1, and receive chemotherapy, are administered the most suitable chemotherapy agents based on their genetic profiles.130 This method aims to enhance the precision and efficacy of chemotherapy while minimizing the risk of adverse effects for patients.
Recent reports suggest that individual polymorphisms have relatively modest effects on clinical outcomes. This shows the need to adopt a more comprehensive method, including polygenetic, phenotypic, epidemiological, and clinical variables to accurately predict the prognosis of NSCLC patients receiving platinum-based chemotherapy.131,132 Based on a previous study conducted in California, a potential therapeutic algorithm for NSCLC has been developed, using ERCC1 gene expression levels.130 The results of the comprehensive dataset including pharmacogenomics study on NSCLC, focusing on platinum-based chemotherapy and essential genes such as ERCC1, ERCC2, ABCC2, and GSTP1, have gained significant attention. Therefore, this systematic review aimed to construct a potential algorithm as a framework for personalized medicine methods in NSCLC therapy.
Challenges and Limitations of SNP Screening in Clinical Practice
Challenges and limitations of SNPs screening in clinical practice include complexities related to result interpretation, standardization of testing methods, and the need for large-scale, diverse datasets to establish robust associations. This is in line with the persistent demand for human resources in the field of genetic interpretation, where education is continually developed. Additionally, integrating SNPs data into routine clinical decision-making processes can be challenging, requiring adequate guidelines and tools for healthcare professionals. Ethical and privacy concerns regarding genetic information, along with cost-effectiveness considerations, pose significant limitations. Although SNPs offer valuable insights, their effectiveness is limited by the entire genetic landscape, requiring a comprehensive method that incorporates multiple genetic and non-genetic factors for a more accurate clinical prognosis. SNPs show complexity, indicating that an impact in one population is not essential for replicating another. This genetic diversity poses a significant challenge, showing the need for studies that are personalized and comprehensive.
Furthermore, ethical and privacy concerns remain a significant barrier to the clinical implementation of pharmacogenomics. Handling sensitive genetic data requires strict adherence to confidentiality, informed consent, and responsible data sharing practices. There is also a risk of genetic discrimination and stigma if data are not managed carefully. As pharmacogenomic testing becomes more accessible, robust regulatory frameworks and ethical guidelines are crucial to ensure patient trust and protect individuals’ rights.
Methodologies and Molecular Technique for SNP Screening
SNPs have significant implications in genetic disease, although recent methods, such as DNA microarrays, qPCR, and sequencing are characterized by intricate procedures. The methods are expensive and apply sophisticated instruments, leading to suboptimal results in clinical settings, particularly regarding multiple SNPs associated with genetic diseases. A previous study conducted in China introduced an innovative point-of-care testing (POCT) system, namely the Amplification Refractory Mutation System (ARMS) coupled with gold magnetic nanoparticles (GMNPs) and lateral flow assay (LFA). This innovation was collectively referred to as the ARMS-LFA system, offering a cost-effective, user-friendly, and highly sensitive method, enabling the uniform detection of multiple SNPs concurrently. The results showed a significant potential as a POCT tool for identifying multiple SNPs correlated with genetic disease.133 Another study on SNP genotyping analysis for clinical applications investigated an oligo-nucleotide array-based method designed for gene-specific SNP genotyping. The results showed that the method, recognized for cost-effectiveness and high-throughput capabilities, had both high sensitivity and a level of accuracy comparable to direct sequencing. To validate accuracy and efficiency, a comparison was made with BRCA1 gene model, in relation to breast and ovarian cancer predisposition.134 Typically, efforts are directed towards identifying and establishing the most significant SNPs as a genetic marker for diagnosis and therapy selection. However, there are situations where the analysis of unidentified SNPs is essential to ensure a comprehensive interpretation. Conventional sequencing, which is highly informative, is often cost-prohibitive. In a recent study, an innovative method for the fluorometric detection of both known and unknown SNPs was introduced based on optimizing the well-established principle of signal loss or gain, using a significantly reduced number of matched or mismatched probes.135
Conclusion
In conclusion, this systematic review showed essential information regarding SNPs in ERCC1, ERCC2, GSTP1, and ABCC2, with their impact on NSCLC therapy outcomes, particularly with platinum-based chemotherapy. Based on the results, ERCC1 rs11615, ERCC2 rs13181, ABCC2 rs717620, and GSTP1 rs1695 were the most frequently investigated SNPs. Among these genes, GSTP1 rs1695 showed significant potential, where 11 studies indicated an association with clinical outcomes and survival in NSCLC patients. Moreover, the integration of SNPs profiling into clinical decision-making substantially improved treatment personalization. By identifying the most appropriate genetic markers, clinicians could optimize therapy selection, enhancing both efficacy and safety. This correlated with the broader trend towards precision medicine in NSCLC, offering opportunities to enhance patient outcomes and minimize adverse effects.
Data Availability Statement
The data available in supplementary material.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Universitas Padjadjaran through the Post Graduate Research Scheme under the Padjadjaran Doctoral Scholarship program for NNA.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Thomas A, Liu SV, Subramaniam DS, Giaccone G. Refining the treatment of NSCLC according to histological and molecular subtypes. Nat Rev Clin Oncol. 2015;12(9):511–526. doi:10.1038/nrclinonc.2015.90
2. Cagle PT, Allen TC, Olsen RJ. Lung cancer biomarkers: present status and future developments. Arch Pathol Lab Med. 2013;137(9):1191–1198. doi:10.5858/arpa.2013-0319-CR
3. Huang Y, Liu L, Cai J, et al. The efficacy and response predictors of platinum-based neoadjuvant chemotherapy in locally advanced cervical cancer. Cancer Manag Res. 2020;12:10469–10477. doi:10.2147/CMAR.S270258
4. Wang Y, Liu ZD, Zhao LM, et al. Individualized treatment of NSCLC: from research to clinical practice. Neoplasma. 2013;60(5):538–545. doi:10.4149/neo_2013_070
5. Amable L. Cisplatin resistance and opportunities for precision medicine. Pharmacol Res. 2016;106:27–36. doi:10.1016/j.phrs.2016.01.001
6. Hicks JK, McLeod HL. Pharmacogenetics and Pharmacogenomics. Elsevier Inc.; 2017; doi:10.1016/B978-0-12-800685-6.00004-7
7. Ma Q, Lu AYH. Pharmacogenetics, Pharmacogenomics, and Individualized Medicine. Clin Pharmacol. 2006;9783709101(2):335–344. doi:10.1007/978-3-7091-0144-5-22
8. Thomas VA, Balthasar JP. Understanding inter-individual variability in monoclonal antibody disposition. Antibodies. 2019;8(4):56. doi:10.3390/antib8040056
9. Organization WH. Executive summary: the selection and use of essential medicines 2021: report of the 23rd WHO Expert Committee on the selection and use of essential medicines: virtual meeting In:
10. Pramesh CS, Badwe RA, Bhoo-Pathy N, et al. Priorities for cancer research in low- and middle-income countries: a global perspective. Nat Med. 2022;28(4):649–657. doi:10.1038/s41591-022-01738-x
11. Patil V, Abraham G, Ravikrishna M, et al. Retrospective analysis: checkpoint inhibitor accessibility for thoracic and head and neck cancers and factors influencing it in a tertiary centre in India. Ecancermedicalscience. 2022:16. doi:10.3332/ecancer.2022.1464.
12. Chan BA, Hughes BGM. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res. 2015;4(1):36–54. doi:10.3978/j.issn.2218-6751.2014.05.01
13. Borcoman E, Nandikolla A, Long G, Goel S, Le Tourneau C. Patterns of response and progression to immunotherapy. Am Soc Clin Oncol Educat Book. 2018;38:169–178. doi:10.1200/EDBK_200643
14. Msc L, Mok KKS, Mok TSK. Developments in targeted therapy & immunotherapy—how non-small cell lung cancer management will change in the next decade: a narrative review. Ann Transl Med. 2023;11(10):358. doi:10.21037/atm-22-4444
15. Rossi A, Di Maio M. Platinum-based chemotherapy in advanced non-small-cell lung cancer: optimal number of treatment cycles. Expert Rev Anticancer Ther. 2016;16(6):653–660. doi:10.1586/14737140.2016.1170596
16. Harada K, Ono S. Background and clinical significance of biomarker-based patient enrichment in non-small-cell lung cancer drug development. Sci Rep. 2024;14(1):7194. doi:10.1038/s41598-024-57556-3
17. Kelly K, Crowley J, Bunn PA, et al. Randomized Phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non–small-cell lung cancer: a southwest oncology group trial. J Clin Oncol. 2001;19(13):3210–3218. doi:10.1200/JCO.2001.19.13.3210
18. Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non–small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. doi:10.1056/NEJMoa011954
19. Sirohi B, Ashley S, Norton A, et al. Early response to platinum-based first-line chemotherapy in non-small cell lung cancer may predict survival. J Thorac Oncol. 2007;2(8):735–740. doi:10.1097/JTO.0b013e31811f3a7d
20. Afifah NN, Diantini A, Intania R, Abdulah R, Barliana MI. Genetic polymorphisms and the efficacy of platinum-based chemotherapy: review. Pharmgenomics Pers Med. 2020;13:427–444. doi:10.2147/PGPM.S267625
21. Magnitude T. Genetic polymorphisms and the efficacy of platinum-based chemotherapy: review. 2018;11–14.
22. Zhang G, Guan Y, Zhao Y, et al. ERCC2/XPD Lys751Gln alter DNA repair efficiency of platinum-induced DNA damage through P53 pathway. Chem Biol Interact. 2017;263:55–65. doi:10.1016/j.cbi.2016.12.015
23. Boldrin E, Malacrida S, Rumiato E, et al. Association between ERCC1rs3212986 and ERCC2/XPDrs1799793 and OS in patients with advanced esophageal cancer. Front Oncol. 2019;9(FEB):1–9. doi:10.3389/fonc.2019.00085
24. Ke HG, Li J, Shen Y, et al. Prognostic significance of GSTP1, XRCC1 and XRCC3 polymorphisms in non-small cell lung cancer patients. Asian Pac J Cancer Prev. 2012;13(9):4413–4416. doi:10.7314/apjcp.2012.13.9.4413
25. Sharma P, Singh N, Sharma S. Impact of ABCB1, ABCC1, ABCC2, and ABCG2 variants in predicting prognosis and clinical outcomes of north Indian lung cancer patients undergoing platinum-based doublet chemotherapy. J Gene Med. 2022;25(1):e3460. doi:10.1002/jgm.3460
26. Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med. 2021;27(8):1345–1356. doi:10.1038/s41591-021-01450-2
27. Jiang W, Cai G, Hu PC, Wang Y. Personalized medicine in non-small cell lung cancer: a review from a pharmacogenomics perspective. Acta Pharm Sin B. 2018;8(4):530–538. doi:10.1016/j.apsb.2018.04.005
28. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. doi:10.1016/j.ijsu.2021.105906
29. Chaplin M, Kirkham JJ, Dwan K, Sloan DJ, Davies G, Jorgensen AL. STrengthening the reporting of pharmacogenetic studies: development of the STROPS guideline. PLoS Med. 2020;17(9):e1003344. doi:10.1371/journal.pmed.1003344
30. Anoushirvani AA, Aghabozorgi R, Ahmadi A, et al. The relationship between rs3212986C>A polymorphism and tumor stage in lung cancer patients. Cureus. 2019;11(4):e4423. doi:10.7759/cureus.4423
31. Li D, Zhou Q, Liu Y, Yang Y, Li Q. DNA repair gene polymorphism associated with sensitivity of lung cancer to therapy. Med Oncol. 2011;29:1622–1628. doi:10.1007/s12032-011-0033-7
32. Isla D, Sarries C, Rosell R, et al. Single nucleotide polymorphisms and outcome in docetaxel-cisplatin-treated advanced non-small-cell lung cancer. Ann Oncol. 2004;15(8):1194–1203. doi:10.1093/annonc/mdh319
33. Bushra MU, Rivu SF, Sifat AE, et al. Genetic polymorphisms of GSTP1, XRCC1, XPC and ERCC1: prediction of clinical outcome of platinum-based chemotherapy in advanced non-small cell lung cancer patients of Bangladesh. Mol Biol Rep. 2020;47(9):7073–7082. doi:10.1007/s11033-020-05771-2
34. Gao H, Ge RC, Liu HY, Wang Y, Yan S. Effect of ERCC1 polymorphism on the response to chemotherapy and clinical outcome of non-small cell lung cancer. Genet Mol Res. 2014;13(4):8997–9004. doi:10.4238/2014.October.31.14
35. Lv H, Han T, Shi X, et al. Genetic polymorphism of GSTP1 and ERCC1 correlated with response to platinum-based chemotherapy in non-small cell lung cancer. Med Oncol. 2014;31(8):86. doi:10.1007/s12032-014-0086-5
36. Shi ZH, Shi GY, Liu LG. Polymorphisms in ERCC1 and XPF gene and response to chemotherapy and overall survival of non-small cell lung cancer. Int J Clin Exp Pathol. 2015;8(3):3132–3137.
37. Zhou C, Ren S, Zhou S, et al. Predictive effects of ERCC1 and XRCC3 SNP on efficacy of platinum-based chemotherapy in advanced NSCLC patients. Jpn J Clin Oncol. 2010;40(10):954–960. doi:10.1093/jjco/hyq071
38. Zhao X, Zhang Z, Yuan Y, Yuan X. Polymorphisms in ERCC1 gene could predict clinical outcome of platinum-based chemotherapy for non-small cell lung cancer patients. Tumour Biol. 2014;35(8):8335–8341. doi:10.1007/s13277-014-2033-7
39. Cheng J, Ha M, Wang Y, et al. A C118T polymorphism of ERCC1 and response to cisplatin chemotherapy in patients with late-stage non-small cell lung cancer. J Cancer Res Clin Oncol. 2011;138(2):231–238. doi:10.1007/s00432-011-1090-1
40. Chen S, Huo X, Lin Y, et al. Association of MDR1 and ERCC1 polymorphisms with response and toxicity to cisplatin-based chemotherapy in non-small-cell lung cancer patients. Int J Hyg Environ Health. 2010;213(2):140–145. doi:10.1016/j.ijheh.2010.01.004
41. Hong W, Wang K, Zhang YP, et al. Methylenetetrahydrofolate reductase C677T polymorphism predicts response and time to progression to gemcitabine-based chemotherapy for advanced non-small cell lung cancer in a Chinese Han population. J Zhejiang Univ Sci B. 2013;14(3):207–215. doi:10.1631/jzus.B1200101
42. Huang SJ, Wang YF, Jin ZY, Sun JY, Guo ZL. Role of ERCC1 variants in response to chemotherapy and clinical outcome of advanced non-small cell lung cancer. Tumour Biol. 2013;35(5):4023–4029. doi:10.1007/s13277-013-1526-0
43. Zhang ZY, Tian X, Wu R, Liang Y, Jin XY. Predictive role of ERCC1 and XPD genetic polymorphisms in survival of Chinese non-small cell lung cancer patients receiving chemotherapy. Asian Pac J Cancer Prev. 2012;13(6):2583–2586. doi:10.7314/apjcp.2012.13.6.2583
44. Ludovini V, Floriani I, Pistola L, et al. Association of cytidine deaminase and xeroderma pigmentosum group D polymorphisms with response, toxicity, and survival in cisplatin/gemcitabine-treated advanced non-small cell lung cancer patients. J Thorac Oncol. 2011;6(12):2018–2026. doi:10.1097/JTO.0b013e3182307e1f
45. Li F, Sun X, Sun N, et al. Association between polymorphisms of ERCC1 and XPD and clinical response to platinum-based chemotherapy in advanced non-small cell lung cancer. Am J Clin Oncol. 2010;33(5):489–494. doi:10.1097/COC.0b013e3181b9cedc
46. Joerger M, Burgers SA, Baas P, et al. Germline polymorphisms in patients with advanced nonsmall cell lung cancer receiving first-line platinum-gemcitabine chemotherapy: a prospective clinical study. Cancer. 2011;118(9):2466–2475. doi:10.1002/cncr.26562
47. Kaewbubpa W, Areepium N, Sriuranpong V. Effect of the ERCC1 (C118T) POLYMORPHISM ON TREATMENT RESPONSE IN ADVANCED NON-SMALL CELL LUNG CANCER PATIENTS UNDERGOING PLATINUM-BASED CHEMotherapy. Asian Pac J Cancer Prev. 2016;17(11):4917–4920. doi:10.22034/APJCP.2016.17.11.4917
48. Tibaldi C, Giovannetti E, Vasile E, et al. Correlation of CDA, ERCC1, and XPD polymorphisms with response and survival in gemcitabine/cisplatin-treated advanced non-small cell lung cancer patients. Clin Cancer Res. 2008;14(6):1797–1803. doi:10.1158/1078-0432.CCR-07-1364
49. Adel Abou Zaghla HM, Abdelhakam DA, Ibrahim Abd el-Mageed A, Ehab Moustafa Ahmed R, Mohsen M. Single nucleotide polymorphism of ERCC1 gene in patients with non-small cell lung cancer and its relation to the response to platinum chemotherapy. 2022. Available from: https://ejhm.journals.ekb.eg/.
50. Li M, Chen R, Ji B, et al. Contribution of XPD and XPF polymorphisms to susceptibility of non-small cell lung cancer in high-altitude areas. Public Health Genomics. 2021;24(3–4):189–198. doi:10.1159/000512641
51. Nairuz T, Bushra YU, Kabir Y. Effect of XPD and TP53 gene polymorphisms on the risk of platinum-based chemotherapy induced toxicity in bangladeshi lung cancer patients. Asian Pac J Cancer Prev. 2021;22(12):3809–3815. doi:10.31557/APJCP.2021.22.12.3809
52. Zhang L, Ma W, Li Y, Wu J, Shi GY. Pharmacogenetics of DNA repair gene polymorphisms in non-small-cell lung carcinoma patients on platinum-based chemotherapy. Genet Mol Res. 2014;13(1):228–236. doi:10.4238/2014.January.14.2
53. Chen X, Sun H, Ren S, et al. Association of XRCC3 and XPD751 SNP with efficacy of platinum-based chemotherapy in advanced NSCLC patients. Clin Transl Oncol. 2012;14(3):207–213. doi:10.1007/s12094-012-0785-3
54. Li XD, Han JC, Zhang YJ, Li HB, Wu XY. Common variations of DNA repair genes are associated with response to platinum-based chemotherapy in NSCLCs. Asian Pac J Cancer Prev. 2013;14(1):145–148. doi:10.7314/apjcp.2013.14.1.145
55. Dong J, Hu Z, Shu Y, et al. Potentially functional polymorphisms in DNA repair genes and non-small-cell lung cancer survival: a pathway-based analysis. Mol, Carcinog. 2011;51(7):546–552. doi:10.1002/mc.20819
56. Liu L, Yuan P, Wu C, et al. Assessment of XPD Lys751Gln and XRCC1 T-77C polymorphisms in advanced non-small-cell lung cancer patients treated with platinum-based chemotherapy. Lung Cancer. 2010;73(1):110–115. doi:10.1016/j.lungcan.2010.11.004
57. Lee SY, Kang HG, Yoo SS, et al. Polymorphisms in DNA repair and apoptosis-related genes and clinical outcomes of patients with non-small cell lung cancer treated with first-line paclitaxel-cisplatin chemotherapy. Lung Cancer. 2013;82(2):330–339. doi:10.1016/j.lungcan.2013.07.024
58. Wu W, Li H, Wang H, et al. Effect of polymorphisms in XPD on clinical outcomes of platinum-based chemotherapy for Chinese non-small cell lung cancer patients. PLoS One. 2012;7(3):e33200. doi:10.1371/journal.pone.0033200
59. Zhou M, Ding YJ, Feng Y, Zhang QR, Xiang Y, Wan HY. Association of xeroderma pigmentosum group D (Asp312Asn, Lys751Gln) and cytidine deaminase (Lys27Gln, Ala70Thr) polymorphisms with outcome in Chinese non-small cell lung cancer patients treated with cisplatin-gemcitabine. Genet Mol Res. 2014;13(2):3310–3318. doi:10.4238/2014.April.29.9
60. Gandara DR, Kawaguchi T, Crowley J, et al. Japanese-US common-arm analysis of paclitaxel plus carboplatin in advanced non-small-cell lung cancer: a model for assessing population-related pharmacogenomics. J Clin Oncol. 2009;27(21):3540–3546. doi:10.1200/JCO.2008.20.8793
61. Cheng H, Qin Q, Sun X, et al. Predictive effect of XPA and XPD polymorphisms on survival of advanced NSCLC patients treated with platinum-based chemotherapy: a three-dimensional (3-D), polyacrylamide gel-based DNA microarray method. Technol Cancer Res Treat. 2013;12(5):473–482. doi:10.7785/tcrt.2012.500337
62. Li Y, Huang XE, Jin GF, Shen HB, Xu L. Lack of any relationship between chemotherapy toxicity in non-small cell lung cancer cases and polymorphisms in XRCC1 codon 399 or XPD codon 751. Asian Pac J Cancer Prev. 2011;12(3):739–742.
63. Li P, Wang YD, Cheng J, Chen JC, Ha MW. Association between polymorphisms of BAG-1 and XPD and chemotherapy sensitivity in advanced non-small-cell lung cancer patients treated with vinorelbine combined cisplatin regimen. Tumor Biol. 2015;36(12):9465–9473. doi:10.1007/s13277-015-3672-z
64. Sun X, Sun N, Chen B, et al. MRP2 and GSTP1 polymorphisms and chemotherapy response in advanced non-small cell lung cancer. Cancer Chemother Pharmacol. 2009;65(3):437–446. doi:10.1007/s00280-009-1046-1
65. Han ZG, Tao J, Yu TT, Shan L. Effect of GSTP1 and ABCC2 polymorphisms on treatment response in patients with advanced non-small cell lung cancer undergoing platinum-based chemotherapy: a study in a Chinese Uygur population. Med Sci Monit. 2017;23:1999–2006. doi:10.12659/MSM.904156
66. Han B, Gao G, Wu W, et al. Association of ABCC2 polymorphisms with platinum-based chemotherapy response and severe toxicity in non-small cell lung cancer patients. Lung Cancer. 2011;72(2):238–243. doi:10.1016/j.lungcan.2010.09.001
67. Qian CY, Wang Y, Zheng Y, et al. Associations of genetic polymorphisms of the transporters organic cation transporter 2 (OCT2), multidrug and toxin extrusion 1 (MATE1), and ATP-binding cassette subfamily C member 2 (ABCC2) with platinum-based chemotherapy response and toxicity in non-sma. Chin J Cancer. 2016;35(1):85. doi:10.1186/s40880-016-0145-8
68. Booton R, Ward T, Heighway J, Ashcroft L, Morris J, Thatcher N. Glutathione-S-transferase P1 isoenzyme polymorphisms, platinum-based chemotherapy, and non-small cell lung cancer. J Thorac Oncol. 2006;1(7):679–683.
69. Wu G, Jiang B, Liu X, Shen Y, Yang S. Association of GSTs gene polymorphisms with treatment outcome of advanced non-small cell lung cancer patients with cisplatin-based chemotherapy. Int J Clin Exp Pathol. 2015;8(10):13346–13352.
70. Liu JY, Liu QM, Li LR. Association of GSTP1 and XRCC1 gene polymorphisms with clinical outcomes of patients with advanced non-small cell lung cancer. Genet Mol Res. 2015;14(3):10331–10337. doi:10.4238/2015.August.28.19
71. Bu L, Zhang LB, Mao X, Wang P. GSTP1 Ile105Val and XRCC1 Arg399Gln gene polymorphisms contribute to the clinical outcome of patients with advanced non-small cell lung cancer. Genet Mol Res. 2016;15(2). doi:10.4238/gmr.15027611
72. Liu K, Lin Q, Ding H, Jin Y, Chen G. Predictive potential role of GSTs gene polymorphisms in the treatment outcome of advanced non-small cell lung cancer patients. Int J Clin Exp Med. 2015;8(11):20918–20924.
73. Zhou F, Yu Z, Jiang T, Lv H, Yao R, Liang J. Genetic polymorphisms of GSTP1 and XRCC1: prediction of clinical outcome of platinum-based chemotherapy in advanced non-small cell lung cancer (NSCLC) patients. Swiss Med Wkly. 2011;141:w13275. doi:10.4414/smw.2011.13275
74. Yuan ZJ, Zhou WW, Liu W, et al. Association of GSTP1 and RRM1 polymorphisms with the response and toxicity of gemcitabine-cisplatin combination chemotherapy in Chinese patients with non-small cell lung cancer. Asian Pac J Cancer Prev. 2015;16(10):4347–4351. doi:10.7314/apjcp.2015.16.10.4347
75. Deng JH, Deng J, Shi DH, Ouyang XN, Niu PG. Clinical outcome of cisplatin-based chemotherapy is associated with the polymorphisms of GSTP1 and XRCC1 in advanced non-small cell lung cancer patients. Clin Transl Oncol. 2015;17(9):720–726. doi:10.1007/s12094-015-1299-6
76. Xiao HL, Yang ZT, Han F, Wei HX. Association of glutathione S-transferase (GST) genetic polymorphisms with treatment outcome of cisplatin-based chemotherapy for advanced non-small cell lung cancer in a Chinese population. Genet Mol Res. 2016;15(2). doi:10.4238/gmr.15027320
77. Jia W, Sun JY, Jia KY, Liu XC. Role of GSTM1, GSTT1, and GSTP1 IIe105Val gene polymorphisms in the response to chemotherapy and overall survival of advanced non-small cell lung cancer. Genet Mol Res. 2016;15(3). doi:10.4238/gmr.15037668
78. de Sousa GF, Wlodarczyk SR, Monteiro G. Carboplatin: molecular mechanisms of action associated with chemoresistance. Braz J Pharm Sci. 2014;50(4):693–702. doi:10.1590/S1984-82502014000400004
79. Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi:10.1016/j.ejphar.2014.07.025
80. Rosell R, Taron M, Ariza A, et al. Molecular predictors of response to chemotherapy in lung cancer. Semin Oncol. 2004;31(1 Suppl 1):20–27. doi:10.1053/j.seminoncol.2003.12.011
81. Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33(1):9–23. doi:10.1016/j.ctrv.2006.09.006
82. Makovec T. Cisplatin and beyond: molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol Oncol. 2019;53(2):148–158. doi:10.2478/raon-2019-0018
83. World Health Organization. Cancer Facts Sheet. World Health Organization. 2022. Available from: https://www.who.int/news-room/fact-sheets/detail/lung-cancer.
84. Srikrishnan A, Fuster MM, Montgrain PR. Curative stereotactic body radiotherapy is safe and effective in early stage non-small cell lung cancer: a single-center retrospective review of a cohort of veterans. Am J Respir Crit Care Med. 2018;197(MeetingAbstracts):1.
85. American Cancer Society. Lung cancer survival rates | 5-year survival rates for lung cancer. Ame Cancer Soc. 2019;2019:7–9.
86. Iyer JG, Blom A, Doumani R, et al. Response rates and durability of chemotherapy among 62 patients with metastaticMerkel cell carcinoma. Cancer Med. 2016;5(9):2294–2301. doi:10.1002/cam4.815
87. Leal F, García-Perdomo HA. Effectiveness of platinum-based chemotherapy in patients with metastatic prostate cancer: systematic review and meta-analysis. Clin Genitourin Cancer. 2019;17(3):e627–e644. doi:10.1016/j.clgc.2019.03.008
88. Petrelli F, Coinu A, Cabiddu M, Ghilardi M, Ardine M, Barni S. Platinum rechallenge in patients with advanced NSCLC: a pooled analysis. Lung Cancer. 2013;81(3):337–342. doi:10.1016/j.lungcan.2013.06.022
89. Chen C, Jiang XL, Zhang CC, Li K. Correlation of genes associated with drug response to prognosis of large cell lung carcinoma. Chin J Cancer. 2011;30(7):497–504. doi:10.5732/cjc.010.10503
90. Karczewski KJ, Francioli LC, Tiao G, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581(7809):434–443. doi:10.1038/s41586-020-2308-7
91. PharmGKB. Clinical annotations and allele frequencies. 2025. Availble from: https://www.pharmgkb.org.
92. Scha OD. Nucleotide excision repair in eukaryotes. 2013;1–19.
93. Rosell R, Taron M, Barnadas A, Scagliotti G, Sarries C, Roig B. Nucleotide excision repair pathways involved in cisplatin resistance in non – small-cell lung cancer. Cancer Control. 2003;10:297–305. doi:10.1177/107327480301000404
94. Moreno V, Gemignani F, Landi S, et al. Polymorphisms in genes of nucleotide and base excision repair: risk and prognosis of colorectal cancer. Clin Cancer Res. 2006;12(7 Pt 1):2101–2108. doi:10.1158/1078-0432.CCR-05-1363
95. Song X, Wang S, Hong X, et al. Single nucleotide polymorphisms of nucleotide excision repair pathway are significantly associated with outcomes of platinum-based chemotherapy in lung cancer. Sci Rep. 2017;7(1):1–11. doi:10.1038/s41598-017-08257-7
96. Xue P, Zhang G, Zhang H, et al. A miR-15a related polymorphism affects NSCLC prognosis via altering ERCC1 repair to platinum-based chemotherapy. J Cell Mol Med. 2022;26(21):5439–5451. doi:10.1111/jcmm.17566
97. Zhang Y, Cao S, Zhuang C, et al. ERCC1 rs11615 polymorphism and chemosensitivity to platinum drugs in patients with ovarian cancer: a systematic review and meta-analysis. J Ovarian Res. 2021;14(1):80. doi:10.1186/s13048-021-00831-y
98. Koutsoukos K, Andrikopoulou A, Dedes N, Zagouri F, Bamias A, Dimopoulos MA. Clinical Perspectives of ERCC1 in Bladder Cancer. Int J Mol Sci. 2020;21(22):8829. doi:10.3390/ijms21228829
99. Zhang H, Li Y, Guo S, et al. Effect of ERCC2 rs13181 and rs1799793 polymorphisms and environmental factors on the prognosis of patients with lung cancer. Am J Transl Res. 2020;12(10):6941–6953.
100. Wu W, Zhang W, Qiao R, et al. Association of XPD polymorphisms with severe toxicity in non–small cell lung cancer patients in a Chinese population. Clin Cancer Res. 2009;15(11):3889–3895. doi:10.1158/1078-0432.CCR-08-2715
101. Zhu ML, He J, Wang M, et al. Potentially functional polymorphisms in the ERCC2 gene and risk of esophageal squamous cell carcinoma in Chinese populations. Sci Rep. 2014;4(1):6281. doi:10.1038/srep06281
102. Yuan P, Miao XP, Zhang XM, et al. XRCC1 and XPD genetic polymorphisms predict clinical responses to platinum-based chemotherapy in advanced non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi. 2006;28(3):196–199.
103. Yang Y, Xian L. The association between the ERCC1/2 polymorphisms and the clinical outcomes of the platinum-based chemotherapy in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis. Tumour Biol. 2013;35(4):2905–2921. doi:10.1007/s13277-013-1493-5
104. Grogan S, Preuss CV. Pharmacokinetics 2021. StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK557744/.
105. Doogue MP, Polasek TM. The ABCD of clinical pharmacokinetics. Ther Adv Drug Saf. 2013;4(1):5–7. doi:10.1177/2042098612469335
106. Berthier J, Arnion H, Saint-Marcoux F, Picard N. Multidrug resistance-associated protein 4 in pharmacology: overview of its contribution to pharmacokinetics, pharmacodynamics and pharmacogenetics. Life Sci. 2019;231(April):116540. doi:10.1016/j.lfs.2019.06.015
107. Han JY, Lim HS, Yoo YK, et al. Associations of ABCB1, ABCC2, and ABCG2 polymorphisms with irinotecan-pharmacokinetics and clinical outcome in patients with advanced non-small cell lung cancer. Cancer. 2007;110(1):138–147. doi:10.1002/cncr.22760
108. Oliveira AL, Rodrigues FFO, Santos RE, et al. GSTT1, GSTM1, and GSTP1 polymorphisms and chemotherapy response in locally advanced breast cancer. Genet Mol Res. 2010;9(2):1045–1053. doi:10.4238/vol9-2gmr726
109. Pincinato EC, Costa EFD, Lopes-Aguiar L, et al. GSTM1, GSTT1 and GSTP1 Ile105Val polymorphisms in outcomes of head and neck squamous cell carcinoma patients treated with cisplatin chemoradiation. Sci Rep. 2019;9(1):9312. doi:10.1038/s41598-019-45808-6
110. Yang Y, Xian L. The association between the GSTP1 A313G and GSTM1 null/present polymorphisms and the treatment response of the platinum-based chemotherapy in non-small cell lung cancer (NSCLC) patients: a meta-analysis. Tumour Biol. 2014;35(7):6791–6799. doi:10.1007/s13277-014-1866-4
111. Campa D, Müller P, Edler L, et al. A comprehensive study of polymorphisms in ABCB1, ABCC2 and ABCG2 and lung cancer chemotherapy response and prognosis. Int, J, Cancer. 2012;131(12):2920–2928. doi:10.1002/ijc.27567
112. Li Z, Xing X, Shan F, et al. ABCC2-24C > T polymorphism is associated with the response to platinum/5-Fu-based neoadjuvant chemotherapy and better clinical outcomes in advanced gastric cancer patients. Oncotarget. 2016;7(34):55449–55457. doi:10.18632/oncotarget.10961
113. De Troia B, Dalu D, Filipazzi V, et al. ABCB1 c.3435C>T polymorphism is associated with platinum toxicity: a preliminary study. Cancer Chemother Pharmacol. 2019;83(4):803–808. doi:10.1007/s00280-019-03794-6
114. Sheehan D, Meade G, Foley VM, Dowd CA. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J. 2001;360(Pt 1):1–16. doi:10.1042/0264-6021:3600001
115. De Luca A, Parker LJ, Ang WH, et al. A structure-based mechanism of cisplatin resistance mediated by glutathione transferase P1-1. Proc Natl Acad Sci U S A. 2019;116(28):13943–13951. doi:10.1073/pnas.1903297116
116. Kim W, Cho YA, Kim DC, Lee KE. Association between genetic polymorphism of GSTP1 and toxicities in patients receiving platinum-based chemotherapy: a systematic review and meta-analysis. Pharmaceuticals. 2022;15(4). doi:10.3390/ph15040439
117. Lecomte T, Landi B, Beaune P, Laurent-Puig P, Loriot MA. Glutathione S-transferase P1 polymorphism (Ile105Val) predicts cumulative neuropathy in patients receiving oxaliplatin-based chemotherapy. Clin Cancer Res. 2006;12(10):3050–3056. doi:10.1158/1078-0432.CCR-05-2076
118. Laechelt S, Turrini E, Ruehmkorf A, Siegmund W, Cascorbi I, Haenisch S. Impact of ABCC2 haplotypes on transcriptional and posttranscriptional gene regulation and function. Pharmacogenomics J. 2011;11(1):25–34. doi:10.1038/tpj.2010.20
119. Liu J, Zhou Y, Liu S, et al. The coexistence of copy number variations (CNVs) and single nucleotide polymorphisms (SNPs) at a locus can result in distorted calculations of the significance in associating SNPs to disease. Hum Genet. 2018;137(6–7):553–567. doi:10.1007/s00439-018-1910-3
120. Ionita-Laza I, Rogers AJ, Lange C, Raby BA, Lee C. Genetic association analysis of copy-number variation (CNV) in human disease pathogenesis. Genomics. 2009;93(1):22–26. doi:10.1016/j.ygeno.2008.08.012
121. Zhang F, Gu W, Hurles ME, Lupski JR. Copy number variation in human health, disease, and evolution. Annu Rev Genomics Hum Genet. 2009;10:451–481. doi:10.1146/annurev.genom.9.081307.164217
122. gnomAD. SNV 19-45420395 - A-G (GRCh38). 2024. Available from: https://gnomad.broadinstitute.org/variant/19-45420395-A-G?dataset=gnomad_r4.
123. Ensembl Asia. Structural variant: nsv3155816. 2024. Available from: https://asia.ensembl.org/Homo_sapiens/StructuralVariation/Mappings?db=core;g=ENSG00000023839;r=10:99782640-99852594;sv=nsv3155816;svf=122984339;vdb=variation.
124. Ensembl Asia. Structural variant: nsv468606. 2024. Available from: https://asia.ensembl.org/Homo_sapiens/StructuralVariation/Mappings?db=core;g=ENSG00000084207;r=11:67583742-67586656;sv=nsv468606;svf=45733391;vdb=variation.
125. Ensembl Asia. Structural variant: nsv555269. 2024. Available from: https://asia.ensembl.org/Homo_sapiens/StructuralVariation/Mappings?db=core;g=ENSG00000084207;r=11:67583742-67586656;sv=nsv555269;svf=121521470;vdb=variation.
126. Ensembl Asia. Structural variant: nsv2785402. 2024. Available from: https://asia.ensembl.org/Homo_sapiens/StructuralVariation/Mappings?db=core;g=ENSG00000104884;r=19:45349837-45370918;sv=nsv2785402;svf=120961317;vdb=variation.
127. Ensembl Asia. Structural variant: nsv3163036. 2024. Available from: https://asia.ensembl.org/Homo_sapiens/StructuralVariation/Mappings?db=core;g=ENSG00000012061;r=19:45407334-45478828;sv=nsv3163036;svf=123050877;vdb=variation.
128. MedGen National Library of Medicine. National Center for Biotechnology Information. Non-small cell lung carcinoma (NSCLC). 2024. Available from: https://www.ncbi.nlm.nih.gov/medgen/C0007131/.
129. gnomAD. SNV 19-45351661 - T-.G (GRCh38). 2024. Available from: https://gnomad.broadinstitute.org/variant/19-45351661-T-G?dataset=gnomad_r4.
130. Gandara DR, Mack PC, Li T, Lara PN, Herbst RS. Evolving treatment algorithms for advanced non-small-cell lung cancer: 2009 looking toward 2012. Chin J Lung Cancer. 2010;13(3):238–241. doi:10.3779/j.issn.1009-3419.2010.03.16
131. Hildebrandt MAT, Gu J, Wu X. Pharmacogenomics of platinum-based chemotherapy in NSCLC. Expert Opin Drug Metab Toxicol. 2009;5(7):745–755. doi:10.1517/17425250902973711
132. Mao CX, Li M, Zhang W, Zhou HH, Yin JY, Liu ZQ. Pharmacogenomics for the efficacy of platinum-based chemotherapy: old drugs, new integrated perspective. Biomed Pharmacother. 2020;126:110057. doi:10.1016/j.biopha.2020.110057
133. Liu X, Zhang C, Liu K, et al. Multiple SNPs detection based on lateral flow assay for phenylketonuria diagnostic. Anal Chem. 2018;90(5):3430–3436. doi:10.1021/acs.analchem.7b05113
134. Monaco A, Menolascina F, Zhao Y, et al. “Sequencing-grade” screening for BRCA1 variants by oligo-arrays. J Transl Med. 2008;6:64. doi:10.1186/1479-5876-6-64
135. Wang E, Adams SD, Zhao Y, et al. A strategy for detection of known and unknown SNP using a minimum number of oligonucleotides applicable in the clinical settings. J Transl Med. 2003;1:4. doi:10.1186/1479-5876-1-4
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

